Wittig reaction
[4] For lithium-free Wittig reactions, studies support a concerted formation of the oxaphosphetane without intervention of a betaine.
In particular, phosphonium ylides 1 react with carbonyl compounds 2 via a [2+2] cycloaddition that is sometimes described as having [π2s+π2a] topology to directly form the oxaphosphetanes 4a and 4b.
Under lithium-free conditions, the stereochemistry of the product 5 is due to the kinetically controlled addition of the ylide 1 to the carbonyl 2.
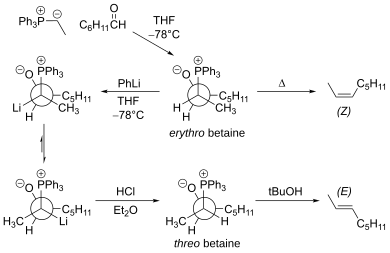
B. Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift".
[9][10] E. Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.
[12] The Wittig reagents generally tolerate carbonyl compounds containing several kinds of functional groups such as OH, OR, nitroarenes, epoxides, and sometimes esters and amides.
In a so-called tandem oxidation-Wittig process the aldehyde is formed in situ by oxidation of the corresponding alcohol.
[15] For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide.