Absorption band
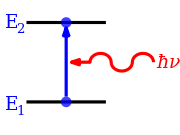
In spectroscopy, an absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum that are characteristic of a particular transition from initial to final state in a substance.
According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states.
In many cases it is convenient to assume that a narrow spectral line is a Lorentzian or Gaussian, depending respectively on the decay mechanism or temperature effects like Doppler broadening.
For charge-transfer complexes and conjugated systems, the band width is complicated by a variety of factors, compared to condensed matter.
[4] Electromagnetic transitions in atoms, molecules and condensed matter mainly take place at energies corresponding to the UV and visible part of the spectrum.
Core electrons in atoms, and many other phenomena, are observed with different brands of XAS in the X-ray energy range.
Electromagnetic transitions in atomic nuclei, as observed in Mössbauer spectroscopy, take place in the gamma ray part of the spectrum.
The main factors that cause broadening of the spectral line into an absorption band of a molecular solid are the distributions of vibrational and rotational energies of the molecules in the sample (and also those of their excited states).