Oxygen
At standard temperature and pressure, two oxygen atoms will bind covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula O2.
Oxygen is too chemically reactive to remain a free element in air without being continuously replenished by the photosynthetic activities of autotrophs such as cyanobacteria, chloroplast-bearing algae and plants.
Oxygen was isolated by Michael Sendivogius before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774.
English chemist John Mayow (1641–1679) refined this work by showing that fire requires only a part of air that he called spiritus nitroaereus.
[12] Robert Hooke, Ole Borch, Mikhail Lomonosov, and Pierre Bayen all produced oxygen in experiments in the 17th and the 18th century but none of them recognized it as a chemical element.
[14] Established in 1667 by the German alchemist J. J. Becher, and modified by the chemist Georg Ernst Stahl by 1731,[15] phlogiston theory stated that all combustible materials were made of two parts.
[10] Highly combustible materials that leave little residue, such as wood or coal, were thought to be made mostly of phlogiston; non-combustible substances that corrode, such as iron, contained very little.
Air did not play a role in phlogiston theory, nor were any initial quantitative experiments conducted to test the idea; instead, it was based on observations of what happens when something burns, that most common objects appear to become lighter and seem to lose something in the process.
[10] Polish alchemist, philosopher, and physician Michael Sendivogius (Michał Sędziwój) in his work De Lapide Philosophorum Tractatus duodecim e naturae fonte et manuali experientia depromti ["Twelve Treatises on the Philosopher's Stone drawn from the source of nature and manual experience"] (1604) described a substance contained in air, referring to it as 'cibus vitae' (food of life,[16]) and according to Polish historian Roman Bugaj, this substance is identical with oxygen.
[17] Sendivogius, during his experiments performed between 1598 and 1604, properly recognized that the substance is equivalent to the gaseous byproduct released by the thermal decomposition of potassium nitrate.
[20] In the meantime, on August 1, 1774, an experiment conducted by the British clergyman Joseph Priestley focused sunlight on mercuric oxide contained in a glass tube, which liberated a gas he named "dephlogisticated air".
After breathing the gas himself, Priestley wrote: "The feeling of it to my lungs was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards.
[22] Chemists (such as Sir Humphry Davy in 1812) eventually determined that Lavoisier was wrong in this regard (e.g. Hydrogen chloride (HCl) is a strong acid that does not contain oxygen), but by then the name was too well established.
For example, Dalton assumed that water's formula was HO, leading to the conclusion that the atomic mass of oxygen was 8 times that of hydrogen, instead of the modern value of about 16.
Oxygen was liquefied in a stable state for the first time on March 29, 1883, by Polish scientists from Jagiellonian University, Zygmunt Wróblewski and Karol Olszewski.
[40] Carotenoids in photosynthetic organisms (and possibly animals) play a major role in absorbing energy from singlet oxygen and converting it to the unexcited ground state before it can cause harm to tissues.
Analysis of a silicon wafer exposed to the solar wind in space and returned by the crashed Genesis spacecraft has shown that the Sun has a higher proportion of oxygen-16 than does the Earth.
The measurement implies that an unknown process depleted oxygen-16 from the Sun's disk of protoplanetary material prior to the coalescence of dust grains that formed the Earth.
Some remote sensing scientists have proposed using the measurement of the radiance coming from vegetation canopies in those bands to characterize plant health status from a satellite platform.
The measurement is technically difficult owing to the low signal-to-noise ratio and the physical structure of vegetation; but it has been proposed as a possible method of monitoring the carbon cycle from satellites on a global scale.
[78] A simplified overall formula for photosynthesis is[79] or simply Photolytic oxygen evolution occurs in the thylakoid membranes of photosynthetic organisms and requires the energy of four photons.
[93] Towards the end of the Carboniferous period (about 300 million years ago) atmospheric O2 levels reached a maximum of 35% by volume,[93] which may have contributed to the large size of insects and amphibians at this time.
[20] Such tankers are used to refill bulk liquid-oxygen storage containers, which stand outside hospitals and other institutions that need large volumes of pure oxygen gas.
Oxygen is also stored and shipped in smaller cylinders containing the compressed gas; a form that is useful in certain portable medical applications and oxy-fuel welding and cutting.
[104][112][113] Normobaric oxygen administration at the highest available concentration is frequently used as first aid for any diving injury that may involve inert gas bubble formation in the tissues.
Pulling on the masks "to start the flow of oxygen" as cabin safety instructions dictate, forces iron filings into the sodium chlorate inside the canister.
This is not a problem except for patients on mechanical ventilators, since gas supplied through oxygen masks in medical applications is typically composed of only 30–50% O2 by volume (about 30 kPa at standard pressure).
Fire and explosion hazards exist when concentrated oxidants and fuels are brought into close proximity; an ignition event, such as heat or a spark, is needed to trigger combustion.
[36] Steel pipes and storage vessels used to store and transmit both gaseous and liquid oxygen will act as a fuel; and therefore the design and manufacture of O2 systems requires special training to ensure that ignition sources are minimized.
[l][144] Liquid oxygen spills, if allowed to soak into organic matter, such as wood, petrochemicals, and asphalt can cause these materials to detonate unpredictably on subsequent mechanical impact.







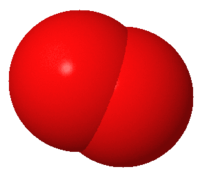



2 .


2 and fixes CO
2 into sugar in what is called a Calvin cycle .

2 build-up in Earth's atmosphere: 1) no O
2 produced; 2) O
2 produced, but absorbed in oceans & seabed rock; 3) O
2 starts to gas out of the oceans, but is absorbed by land surfaces and formation of ozone layer; 4–5) O
2 sinks filled and the gas accumulates




2 is used in space suits .

2 is used to smelt and/or decarburize iron .

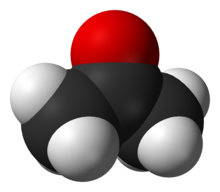
2 O ) is the most familiar oxygen compound.




2 at higher than normal pressure and a spark led to a fire and the loss of the Apollo 1 crew.