Acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes.
[1] Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain.
[18] Additionally, recurrent infections, feeling tired, arm or leg pain, and enlarged lymph nodes can be prominent features.
[19] Central nervous system (CNS) symptoms such as cranial neuropathies due to meningeal infiltration are identified in less than 10% of adults and less than 5% of children, particularly mature B-cell ALL (Burkitt leukemia) at presentation.
[4] The uneven distribution of genetic risk factors may help explain differences in disease rates among ethnic groups.
[6] Evidence suggests that secondary leukemia can develop in individuals treated with certain types of chemotherapy, such as epipodophyllotoxins and cyclophosphamide.
[6][4] The delayed-infection hypothesis states that ALL results from an abnormal immune response to infection in a person with genetic risk factors.
Delayed development of the immune system due to limited disease exposure may result in excessive production of lymphocytes and increased mutation rate during an illness.
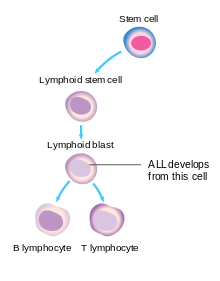
These lymphoblasts build up in the bone marrow and may spread to other sites in the body, such as lymph nodes, the mediastinum, the spleen, the testicles, and the brain, leading to the common symptoms of the disease.
Brain and spinal column involvement can be diagnosed either through confirmation of leukemic cells in the lumbar puncture or through clinical signs of CNS leukemia as described above.
TdT is a protein expressed early in the development of pre-T and pre-B cells, whereas CALLA is an antigen found in 80% of ALL cases and also in the "blast crisis" of CML.
[42][43] While some clinicians still use the FAB scheme to describe tumor cell appearance, much of this classification has been abandoned because of its limited impact on treatment choice and prognostic value.
Must monitor closely for tumor lysis syndrome after initiating therapy Monitoring the initial response to treatment is important as failure to show clearance of blood or bone marrow blasts within the first two weeks of therapy has been associated with a higher risk of relapse Start CNS prophylaxis and administer intrathecal chemotherapy via Ommaya reservoir or multiple lumbar punctures Central nervous system prophylaxis can be achieved via:[50] In Philadelphia chromosome-positive ALL, the intensity of initial induction treatment may be less than has been traditionally given.
B-cell ALL is often associated with cytogenetic abnormalities (specifically, t(8;14), t (2;8), and t(8;22)), which require aggressive therapy consisting of brief, high-intensity regimens.
Additionally, tyrosine kinase inhibitors (TKIs) such as imatinib and dasatinib are incorporated for Philadelphia chromosome-positive ALL, improving treatment outcomes.
[53] Radiation therapy (or radiotherapy) is used on painful bony areas, in high disease burdens, or as part of the preparations for a bone marrow transplant (total body irradiation).
Recent studies showed that CNS chemotherapy provided results as favorable but with fewer developmental side effects.
However, this subtype of ALL is frequently resistant to the combination of chemotherapy and TKIs, and allogeneic stem cell transplantation is often recommended upon relapse.
This technology uses a single chain variable fragment (scFv) designed to recognize the cell surface marker CD19 as a method of treating ALL.
Pseudotyped, self-inactivating lentiviruses are an effective method for the stable insertion of a desired transgene into the target cell.
[medical citation needed] In 2017, tisagenlecleucel was approved by the FDA as a CAR-T therapy for people with acute B-cell lymphoblastic leukaemia who did not respond adequately to other treatments or have relapsed.
[64][65] Typically, people who experience a relapse in their ALL after initial treatment have a poorer prognosis than those who remain in complete remission after induction therapy.
[66] Low-dose palliative radiation may also help reduce the burden of tumors inside or outside the central nervous system and alleviate some symptoms.
[70] Brexucabtagene autoleucel (Tecartus) was approved by the FDA in October 2021 for the treatment of adults with relapsed or refractory B-cell precursor ALL, and later by the EMA in December 2021.
[71][72][73] Each dose of brexucabtagene autoleucel is a customized treatment created using the recipient's immune system to help fight the leukaeamia.
[74] Before the development of chemotherapy regimens and hematopoietic stem cell transplants, children were surviving a median length of 3 months, largely due to either infection or bleeding.
Since the advent of chemotherapy, the prognosis for childhood leukemia has improved greatly and children with ALL are estimated to have a 95% probability of achieving a successful remission after 4 weeks of initiating treatment.
It is estimated that 60–80% of adults undergoing induction chemotherapy achieve complete remission after 4 weeks, and those over the age of 70 have a cure rate of 5%.
[48][75] However, there are differing prognoses for ALL among individuals depending on a variety of factors: T-ALL (children) >1 week to clear blasts from blood <1 week to clear blasts from blood Cytogenetics, the study of characteristic large changes in the chromosomes of cancer cells, is an important predictor of outcome.
Acute leukemias normally require prompt, aggressive treatment, despite significant risks of pregnancy loss and birth defects, especially if chemotherapy is given during the developmentally sensitive first trimester.