Green–Davies–Mingos rules
In organometallic chemistry, the Green–Davies–Mingos rules predict the regiochemistry for nucleophilic addition to 18-electron metal complexes containing multiple unsaturated ligands.
[1] The rules were published in 1978 by organometallic chemists Stephen G. Davies, Malcolm Green, and Michael Mingos.
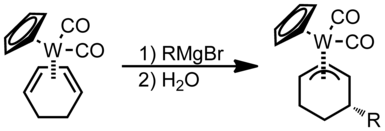
Nucleophilic addition does not occur if kCO* (the effective force constant for the CO ligand) is below a threshold value [2] The following figure shows a ligated metal attached to a carbonyl group.
Incoming nucleophilic attack happens at one of the termini of the π-system in the figure below: In this example the ring system can be thought of as analogous to 1,3-butadiene.
Following the Green–Davies–Mingos rules, since butadiene is an open π-ligand of even hapticity, nucleophilic attack will occur at one of the terminal positions of the π-system.