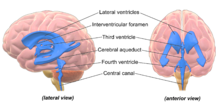
Aging brain
The STAC model looks at factors like neural changes to the white matter, dopamine depletion, shrinkage, and cortical thinning.
One proposed mechanism for the observed age-related plasticity deficits in animals is the result of age-induced alterations in calcium regulation.
Evidence in support of this idea from animal work has also suggested that this cognitive deficit is due to functional and biochemical factors such as changes in enzymatic activity, chemical messengers, or gene expression in cortical circuits.
[12] Advances in MRI technology have provided the ability to see the brain structure in great detail in an easy, non-invasive manner in vivo.
[13] Studies using Voxel-based morphometry have identified areas such as the insula and superior parietal gyri as being especially vulnerable to age-related losses in grey matter of older adults.
[13] Sowell et al., reported that the first 6 decades of an individual's life were correlated with the most rapid decreases in grey matter density, and this occurred over dorsal, frontal, and parietal lobes on both interhemispheric and lateral brain surfaces.
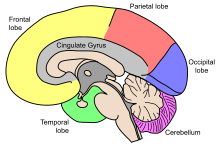
It is also worth noting that areas such as the cingulate gyrus, and occipital cortex surrounding the calcarine sulcus appear exempt from this decrease in grey matter density over time.
[20] In normal, non-demented aging, the number of tangles in each affected cell body is relatively low[20] and restricted to the olfactory nucleus, parahippocampal gyrus, amygdala and entorhinal cortex.
This DNA damage includes the oxidized nucleoside 8-hydroxydeoxyguanosine (8-OHdG), single- and double-strand breaks, DNA-protein cross-links and malondialdehyde adducts (reviewed in Bernstein et al.[26]).
[27] Lu et al.[28] studied the transcriptional profiles of the human frontal cortex of individuals ranging from 26 to 106 years of age.
They concluded that DNA damage may reduce the expression of selectively vulnerable genes involved in learning, memory and neuronal survival, initiating a pattern of brain aging that starts early in life.
[29] According to a review, neuroinflammatory changes, "including microglial activation and production of inflammatory cytokines", occur with normal aging.
Several studies have identified a number of these neurotransmitters, as well as their receptors, that exhibit a marked alteration in different regions of the brain as part of the normal aging process.
Studies using positron emission tomography (PET) in living human subjects have shown a significant age-related decline in dopamine synthesis,[34] notably in the striatum and extrastriatal regions (excluding the midbrain).
Studies conducted using PET methods on humans, in vivo, show that levels of the 5-HT2 receptor in the caudate nucleus, putamen, and frontal cerebral cortex, decline with age.
Similarly, one might expect older adults to do poorly on tasks of sustained attention, which measure the ability to attend to and respond to stimuli for an extended period of time.
Results suggest that sustained attention increases in early adulthood and then remains relatively stable, at least to the middle of the eighth decade of life.
[62][63] Studies indicate late-stage aging, and/or late-life dementias,[62] decreases behavioral flexibility and impair deliberation about courses of action.
[28] Genes that are down-regulated over the age of 40 include:[citation needed] Genes that are upregulated include:[citation needed] The cerebellum is the youngest brain region (and probably body part) in centenarians according to an epigenetic biomarker of tissue age known as epigenetic clock: it is about 15 years younger than expected in a centenarian.
[22][69] This hypothesis suggests that two patients might have the same brain pathology, with one person experiencing noticeable clinical symptoms, while the other continues to function relatively normally.
[89] Longitudinal research studies have recently conducted genetic analyses of centenarians and their offspring to identify protective factors against the negative effects of aging.
[90] Specifically, valine CETP homozygotes but not heterozygotes experienced a relative 51% less decline in memory compared to a reference group after adjusting for demographic factors and APOE status.
[92] A study found that myeloid cells are drivers of a maladaptive inflammation element of brain-ageing in mice and that this can be reversed or prevented via inhibition of their EP2 signalling.
Differences in cognitive aging might be tied to the lack of or reduced access to medical care and, as a result, suffer disproportionately from negative health outcomes.
[citation needed] In the United States, Black and African American demographics disproportionately experience metabolic dysfunction with age.
Metabolite profiles of the healthy aging index - a score that assesses neurocognitive function, among other correlates of health through the years - are associated with cardiovascular disease.
Reviews of current literature studying natives in Australia, Brazil, Canada, and the United States from participants aged 45 to 94 years old reveal varied prevalence rates for cognitive impairment not related to dementia, from 4.4% to 17.7%.
[104] These results can be interpreted in the context of culturally biased neurocognitive tests, preexisting health conditions, poor access to healthcare, lower educational attainment, and/or old age.
[105] Compared to their male counterparts, women's scores on the mini–mental state examination (MMSE) tend to decline at slightly faster rates with age.
[109] This may be because families of higher socioeconomic status (SES) are equipped to provide their children with resources early on to facilitate cognitive development.


As part of the reward pathway , dopamine is manufactured in nerve cell bodies located within VTA and is released in the nucleus accumbens and the prefrontal cortex . The motor functions of dopamine are linked to a separate pathway, with cell bodies in the substantia nigra that manufacture and release dopamine into the striatum .