Alfaxalone
[1][2][3] Though it is more expensive than other induction agents,[4] it often preferred due to the lack of depressive effects on the cardiovascular system.
It is cleared quickly by the liver, giving it a relatively short terminal half-life and preventing it from accumulating in the body, lowering the chance of overdose.
[5] While it is commonly used in cats and dogs, it has also been successfully used in rabbits,[6] horses, sheep, pigs, and exotics such as red-eared turtles, axolotl, green iguanas, marmosets,[7] and koi fish.
[8] As an induction agent, alfaxalone causes the animal to relax enough to be intubated, which then allows the administration of inhalational anesthesia.
[14] The cyclodextrin is a large, starch-derived molecule with a hydrophobic core where alfaxalone stays, allowing the mixture to be dissolved in water and sold as an aqueous solution.
[17] Alfaxalone should be administered slowly over a period of at least 60 seconds or until anesthesia is induced, as quick administration increases the risk of apnea.
[5][13] Alfaxalone has some depressive effects on the central nervous system, including a reduction in cerebral blood flow, intracranial pressure, and body temperature.
[17] Greyhounds, who are particularly susceptible to anesthetic side effects, can have decreased blood flow and oxygen supply to the liver.
[4][13] Dogs and cats will paddle in the air, vocalize excessively, may remain rigid or twitch, and have exaggerated reactions to external stimuli such as light and noise.
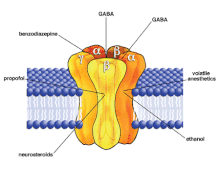
[20] It binds to the M3/M4 domains of the α subunit and allosterically modifies the receptor to facilitate the movement of chloride ions into the cell, resulting in hyperpolarization of the post-synaptic nerve (which inhibits actions potentials).
[17][22] Alfaxalone, however, does not share the benzodiazepine binding site,[23] and actually prefers different GABAA receptors than benzodiazepenes do.
[11] Research suggests that neuroactive steroids increase the expression of GABAA receptors, making it more difficult to build tolerance.
[22] Alfaxalone is metabolized quickly and does not accumulate in the body; its use as an induction agent thus doesn't increase the time needed to recover from anesthesia.
[14] Alfaxalone is the INNTooltip International Nonproprietary Name, BANTooltip British Approved Name, DCFTooltip Dénomination Commune Française, and JANTooltip Japanese Accepted Name of alfaxolone.
[14][32] Alfaxalone is marketed for veterinary use under the brand name Alfaxan in a number of countries, including Australia, Belgium, Canada, France, Germany, Ireland, Japan, the Netherlands, New Zealand, South Africa, South Korea, Spain, Taiwan, the United Kingdom, and the United States.