Progesterone
Progesterone (/proʊˈdʒɛstəroʊn/ ⓘ; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species.
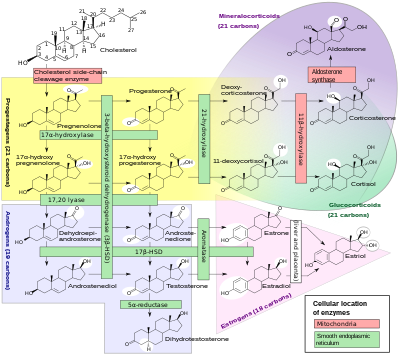
It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.
As a potent agonist of the nuclear progesterone receptor (nPR) (with an affinity of KD = 1 nM) the resulting effects on ribosomal transcription plays a major role in regulation of female reproduction.
[19] It also functions as a ligand of the PGRMC1 (progesterone receptor membrane component 1) which impacts tumor progression, metabolic regulation, and viability control of nerve cells.
[25] Progesterone prevents MR activation by binding to this receptor with an affinity exceeding even those of aldosterone and glucocorticoids such as cortisol and corticosterone,[25] and produces antimineralocorticoid effects, such as natriuresis, at physiological concentrations.
[26] In addition, progesterone binds to and behaves as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol).
[27][28] Progesterone, through its neurosteroid active metabolites such as 5α-dihydroprogesterone and allopregnanolone, acts indirectly as a positive allosteric modulator of the GABAA receptor.
As a result, it has been suggested that substances that block the progesterone binding site on CatSper channels could potentially be used in male contraception.
[40][41][42] Elevated levels of progesterone potently reduce the sodium-retaining activity of aldosterone, resulting in natriuresis and a reduction in extracellular fluid volume.
Progesterone, produced by the placenta during pregnancy, plays a role in fetal sexual differentiation by serving as a precursor molecule for the synthesis of DHT via the backdoor pathway.
In the absence of adequate levels of steroidogenic enzymes during fetal development, the backdoor pathway for DHT synthesis can become deficient, leading to undermasculinization of the male fetus.
[44] Progesterone has key effects via non-genomic signalling on human sperm as they migrate through the female reproductive tract before fertilization occurs, though the receptor(s) as yet remain unidentified.
In conjunction with prolactin, it mediates lobuloalveolar maturation of the mammary glands during pregnancy to allow for milk production and thus lactation and breastfeeding of offspring following parturition (childbirth).
[40][41][42] It has been found that RANKLTooltip Receptor activator of nuclear factor kappa-B ligand is a critical downstream mediator of progesterone-induced lobuloalveolar maturation.
[63][64] Most progestins, or synthetic progestogens, like medroxyprogesterone acetate, have been found to increase the risk of breast cancer in postmenopausal people in combination with estrogen as a component of menopausal hormone therapy.
[70] Hormone replacement therapy, consisting of systemic treatment with estrogen alone or in combination with a progestogen, has well-documented and considerable beneficial effects on the skin of postmenopausal people.
[70] In addition, a study has found that topical 2% progesterone cream significantly increases skin elasticity and firmness and observably decreases wrinkles in peri- and postmenopausal people.
It does so by inhibiting enzymes involved in the apoptosis pathway specifically concerning the mitochondria, such as activated caspase 3 and cytochrome c.[85] Not only does progesterone help prevent further damage, it has also been shown to aid in neuroregeneration.
Animal studies show that progesterone treatment leads to a decrease in edema levels by increasing the concentration of macrophages and microglia sent to the injured tissue.
[84] There is also evidence that the addition of progesterone can also help remyelinate damaged axons due to trauma, restoring some lost neural signal conduction.
Between 7 and 9 weeks, the placenta begins to produce progesterone in place of the corpus luteum in a process called the luteal-placental shift.
For the example, cortisone can be simultaneously deoxygenated at the C-17 and C-21 position by treatment with iodotrimethylsilane in chloroform to produce 11-keto-progesterone (ketogestin), which in turn can be reduced at position-11 to yield progesterone.
[161] An economical semisynthesis of progesterone from the plant steroid diosgenin isolated from yams was developed by Russell Marker in 1940 for the Parke-Davis pharmaceutical company.
The key step of the synthesis is the π-cation cyclization of 13 in which the B-, C-, and D-rings of the steroid are simultaneously formed to produce 14.
Finally, the diketone 17 undergoes an intramolecular aldol condensation by treating with aqueous potassium hydroxide to produce progesterone.
[17][171] This was achieved by Adolf Butenandt at the Chemisches Institut of Technical University in Danzig, who extracted this new compound from several thousand liters of urine.
[171][174] Up to this point, progesterone, known generically as corpus luteum hormone, had been being referred to by several groups by different names, including corporin, lutein, luteosterone, and progestin.
[17][175] In 1935, at the time of the Second International Conference on the Standardization of Sex Hormones in London, England, a compromise was made between the groups and the name progesterone (progestational steroidal ketone) was created.
The rationale for using progesterone tests is that increased numbers begin in close proximity to preovulatory surge in gonadotrophins and continue through ovulation and estrus.
Pricing for progesterone can vary depending location, insurance coverage, discount coupons, quantity, shortages, manufacturers, brand or generic versions, different pharmacies, and so on.





• The ranges denoted By biological stage may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle.
• The ranges denoted Inter-cycle variability are more appropriate to use in non-monitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population.
• The ranges denoted Inter-woman variability are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.



