Alkaline battery
[1][2][3][4][5] Alkaline batteries contain zinc (Zn) and manganese dioxide (MnO2) (Health codes 1), which is a cumulative neurotoxin and can be toxic in higher concentrations.
[6] Alkaline batteries are used in many household items such as Portable media players, digital cameras, toys, flashlights, and radios.
Batteries with alkaline (rather than acid) electrolyte were first developed by Waldemar Jungner in 1899, and, working independently, Thomas Edison in 1901.
[9] When alkaline batteries were introduced in the late 1960s, their zinc electrodes (in common with the then ubiquitous carbon-zinc cells) had a surface film of mercury amalgam.
The concentration of alkaline electrolyte of potassium hydroxide remains constant, as there are equal amounts of OH− anions consumed and produced in the two half-reactions occurring at the electrodes.
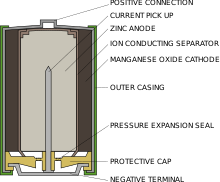
The positive electrode mixture is a compressed paste of manganese dioxide with carbon powder added for increased conductivity.
The hollow center of the cathode is lined with a separator, which prevents contact of the electrode materials and short-circuiting of the cell.
[17] The cell is then wrapped in aluminium foil, a plastic film, or rarely, cardboard, which acts as a final layer of leak protection as well as providing a surface on which logos and labels can be printed.
Attempts to recharge standard alkaline batteries may cause rupture, or the leaking of hazardous liquids that corrode the equipment.
However, it is reported that standard alkaline batteries can often be recharged a few times (typically not more than ten), albeit with reduced capacity after each charge; chargers are available commercially.
[19] In 2017 Gautam G. Yadav published papers reporting that alkaline batteries made by interleaving the interlayers with copper ions could be recharged for over 6,000 cycles due to the theoretical second electron capacity of manganese dioxide.
[clarification needed][20][21] The energy density of these rechargeable batteries with copper intercalated manganese dioxide is reported to be over 160 Wh/L, the best among the aqueous-based chemistries.
[20] Alkaline batteries are prone to leaking potassium hydroxide, a caustic agent that can cause respiratory, eye and skin irritation.
Applying reverse current (such as by recharging disposable-grade cells, or by mixing batteries of different types or state of charge in the same device) can increase the risk of leakage.
The reason for leaks is that as batteries discharge – either through usage or gradual self-discharge – the chemistry of the cells changes and some hydrogen gas is generated.
In addition, as the battery ages, its steel outer canister may gradually corrode or rust, which can further contribute to containment failure.
Once a leak has formed due to corrosion of the outer steel shell, potassium hydroxide absorbs carbon dioxide from the air to form a feathery crystalline structure of potassium carbonate that grows and spreads out from the battery over time, following along metal electrodes to circuit boards where it commences oxidation of copper tracks and other components, leading to permanent circuitry damage.