Alkaloid
[2] Alkaloids are produced by a large variety of organisms including bacteria, fungi, plants, and animals.
[4] Alkaloids have a wide range of pharmacological activities including antimalarial (e.g. quinine), antiasthma (e.g. ephedrine), anticancer (e.g. homoharringtonine),[5] cholinomimetic (e.g. galantamine),[6] vasodilatory (e.g. vincamine), antiarrhythmic (e.g. quinidine), analgesic (e.g. morphine),[7] antibacterial (e.g. chelerythrine),[8] and antihyperglycemic activities (e.g.
Other alkaloids possess psychotropic (e.g. psilocin) and stimulant activities (e.g. cocaine, caffeine, nicotine, theobromine),[11] and have been used in entheogenic rituals or as recreational drugs.
[12] Although alkaloids act on a diversity of metabolic systems in humans and other animals, they almost uniformly evoke a bitter taste.
[nb 1] However, the term came into wide use only after the publication of a review article, by Oscar Jacobsen in the chemical dictionary of Albert Ladenburg in the 1880s.
[17] Where several alkaloids are extracted from one plant their names are often distinguished by variations in the suffix: "idine", "anine", "aline", "inine" etc.
[30] The Odyssey of Homer referred to a gift given to Helen by the Egyptian queen, a drug bringing oblivion.
[31] A Chinese book on houseplants written in 1st–3rd centuries BC mentioned a medical use of ephedra and opium poppies.
In 1804, the German chemist Friedrich Sertürner isolated from opium a "soporific principle" (Latin: principium somniferum), which he called "morphium", referring to Morpheus, the Greek god of dreams; in German and some other Central-European languages, this is still the name of the drug.
Several other alkaloids were discovered around that time, including xanthine (1817), atropine (1819), caffeine (1820), coniine (1827), nicotine (1828), colchicine (1833), sparteine (1851), and cocaine (1860).
[38] Initially, when knowledge of chemical structures was lacking, botanical classification of the source plants was relied on.
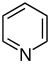
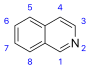
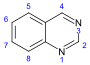
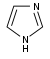
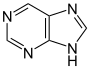
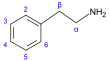
[17][39] More recent classifications are based on similarity of the carbon skeleton (e.g., indole-, isoquinoline-, and pyridine-like) or biochemical precursor (ornithine, lysine, tyrosine, tryptophan, etc.).
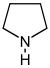
[17] However, they require compromises in borderline cases;[38] for example, nicotine contains a pyridine fragment from nicotinamide and a pyrrolidine part from ornithine[40] and therefore can be assigned to both classes.
Exceptions include scopolamine hydrobromide, which is soluble in organic solvents, and the water-soluble quinine sulfate.
Depending on the type of plants, the maximum concentration is observed in the leaves (for example, black henbane), fruits or seeds (Strychnine tree), root (Rauvolfia serpentina) or bark (cinchona).
[175] Beside plants, alkaloids are found in certain types of fungus, such as psilocybin in the fruiting bodies of the genus Psilocybe, and in animals, such as bufotenin in the skin of some toads[24] and a number of insects, markedly ants.
[179] Most methods exploit the property of most alkaloids to be soluble in organic solvents[4] but not in water, and the opposite tendency of their salts.
Then, the impurities are dissolved by weak acids; this converts alkaloid bases into salts that are washed away with water.
[186] Tracking and dosing the extracted solenopsin ant alkaloids has been described as possible based on their absorbance peak around 232 nanometers.
[135][195] Alkaloids are among the most important and best-known secondary metabolites, i.e. biogenic substances not directly involved in the normal growth, development, or reproduction of the organism.
Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity.
[200] An early hypothesis, that alkaloids are the final products of nitrogen metabolism in plants, as urea and uric acid are in mammals, was refuted by the finding that their concentration fluctuates rather than steadily increasing.
This moth feeds on its highly toxic and alkaloid-rich host plant poison hemlock (Conium maculatum) during its larval stage.
[205] Medical use of alkaloid-containing plants has a long history, and, thus, when the first alkaloids were isolated in the 19th century, they immediately found application in clinical practice.
[209] Prior to the development of a wide range of relatively low-toxic synthetic pesticides, some alkaloids, such as salts of nicotine and anabasine, were used as insecticides.