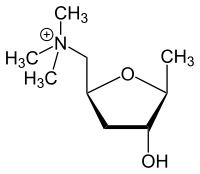
Muscarine
Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol.
The name muscarine derives from that of Amanita muscaria, from which it was first isolated, by German chemists Oswald Schmiedeberg and Richard Koppe at the University of Tartu, who reported their findings in 1869.
Muscarine was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in circulatory collapse and death.
Being a quaternary ammonium salt, muscarine is less completely absorbed from the gastrointestinal tract than tertiary amines, and it does not cross the blood–brain barrier.
The scheme below represents a very efficient way of the synthesis of (+)-muscarine according to the scientists Chan and Li in the Canadian journal of Chemistry in 1992.
Treatment of the crude aldehyde with allyl bromide and zinc powder in water with NH4Cl as catalyst resulted in an anti:syn mixture of 5a and 5b.
The odd numbered receptors, M1, M3 and M5, interact with Gq proteins to stimulate phosphoinositide hydrolysis and the release of intracellular calcium.
Conversely, the even numbered receptors, M2 and M4, interact with Gi proteins to inhibit adenylyl cyclase, which results in a decrease of intracellular concentration of cyclic adenosine monophosphate (cAMP).
[19] Muscarinic agonists are used as drugs in treating glaucoma, postoperative ileus, congenital megacolon, urinary retention and xerostomia.
Cardiac ventricles contain muscarinic receptors that mediate a decrease in the force of contractions leading to a lower blood pressure.
Bronchoconstriction leads to asthmatic attacks and severe dyspnea, and bradycardia combined with marked hypotension and vasodilation results in circulatory shock.
Death after 8 to 9 hours has been reported in about 5% of the cases, but can be avoided completely by prompt administration of IV or IM anticholinergic drugs.