Aluminium
Aluminium is found on Earth primarily in rocks in the crust, where it is the third-most abundant element, after oxygen and silicon, rather than in the mantle, and virtually never as the free metal.
[18] Most meteorite scientists believe that the energy released by the decay of 26Al was responsible for the melting and differentiation of some asteroids after their formation 4.55 billion years ago.
[29] Aluminium is not as strong or stiff as steel, but the low density makes up for this in the aerospace industry and for many other applications where light weight and relatively high strength are crucial.
[25] Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and bonding in aluminium compounds tends towards covalency;[39] this behavior is similar to that of beryllium (Be2+), and the two display an example of a diagonal relationship.
[39][44] Because of its general resistance to corrosion, aluminium is one of the few metals that retains silvery reflectance in finely powdered form, making it an important component of silver-colored paints.
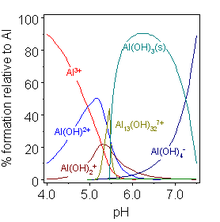
[16] Such solutions are acidic as this cation can act as a proton donor and progressively hydrolyze until a precipitate of aluminium hydroxide, Al(OH)3, forms.
[39] This behavior of Al(OH)3 is termed amphoterism and is characteristic of weakly basic cations that form insoluble hydroxides and whose hydrated species can also donate their protons.
All three are prepared by direct reaction of their elements at about 1,000 °C (1,800 °F) and quickly hydrolyze completely in water to yield aluminium hydroxide and the respective hydrogen chalcogenide.
26Al was present in the early Solar System with abundance of 0.005% relative to 27Al but its half-life of 728,000 years is too short for any original nuclei to survive; 26Al is therefore extinct.
This is because aluminium easily forms the oxide and becomes bound into rocks and stays in the Earth's crust, while less reactive metals sink to the core.
[70] Native aluminium metal is extremely rare and can only be found as a minor phase in low oxygen fugacity environments, such as the interiors of certain volcanoes.
[75] After the Crusades, alum, an indispensable good in the European fabric industry,[76] was a subject of international commerce;[77] it was imported to Europe from the eastern Mediterranean until the mid-15th century.
[81] In 1754, German chemist Andreas Sigismund Marggraf synthesized alumina by boiling clay in sulfuric acid and subsequently adding potash.
[96] As large-scale production caused aluminium prices to drop, the metal became widely used in jewelry, eyeglass frames, optical instruments, tableware, and foil, and other everyday items in the 1890s and early 20th century.
[118] In 2021, prices for industrial metals such as aluminium have soared to near-record levels as energy shortages in China drive up costs for electricity.
[123] British chemist Humphry Davy, who performed a number of experiments aimed to isolate the metal, is credited as the person who named the element.
The first name proposed for the metal to be isolated from alum was alumium, which Davy suggested in an 1808 article on his electrochemical research, published in Philosophical Transactions of the Royal Society.
One example was Essai sur la Nomenclature chimique (July 1811), written in French by a Swedish chemist, Jöns Jacob Berzelius, in which the name aluminium is given to the element that would be synthesized from alum.
Their usage is currently regional: aluminum dominates in the United States and Canada; aluminium is prevalent in the rest of the English-speaking world.
[131] In 1812, British scientist Thomas Young[132] wrote an anonymous review of Davy's book, in which he proposed the name aluminium instead of aluminum, which he thought had a "less classical sound".
[134] Ludwig Wilhelm Gilbert had proposed Thonerde-metall, after the German "Thonerde"[l] for alumina, in his Annalen der Physik but that name never caught on at all even in Germany.
It is unknown whether this spelling was introduced by mistake or intentionally, but Hall preferred aluminum since its introduction because it resembled platinum, the name of a prestigious metal.
Small crystals of aluminium hydroxide are collected to serve as seeding agents; coarse particles are converted to alumina by heating; the excess solution is removed by evaporation, (if needed) purified, and recycled.
In this energy-intensive process, a solution of alumina in a molten (940 and 970 °C (1,720 and 1,780 °F)) mixture of cryolite (Na3AlF6) with calcium fluoride is electrolyzed to produce metallic aluminium.
[147] Recycling involves melting the scrap, a process that requires only 5% of the energy used to produce aluminium from ore, though a significant part (up to 15% of the input material) is lost as dross (ash-like oxide).
[187][188] Evidence published in 1989 indicates that, for Alzheimer's patients, aluminium may act by electrostatically crosslinking proteins, thus down-regulating genes in the superior temporal gyrus.
[189] Aluminium, although rarely, can cause vitamin D-resistant osteomalacia, erythropoietin-resistant microcytic anemia, and central nervous system alterations.
[190] Aluminium has been suspected of being a possible cause of Alzheimer's disease,[191] but research into this for over 40 years has found, as of 2018[update], no good evidence of causal effect.
sur la Physique 19 378) < classical Latin alūmin-, alūmen alum n.1, after French -ine -ine suffix4.Potassium, acting upon alumine and glucine, produces pyrophoric substances of a dark grey colour, which burnt, throwing off brilliant sparks, and leaving behind alkali and earth, and which, when thrown into water, decomposed it with great violence.
From the beginning of the 20th cent., aluminum gradually became the predominant form in North America; it was adopted as the official name of the metal in the United States by the American Chemical Society in 1925.