Amantadine
Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended because of widespread drug resistance.
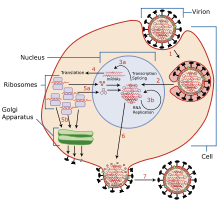
[11][12] The antiviral mechanism of action is inhibition of the influenza virus A M2 proton channel, which prevents endosomal escape (i.e., the release of viral genetic material into the host cytoplasm).
[18] Other uses include treatment of drug-induced extrapyramidal side effects, motor fluctuations during levodopa therapy in Parkinson's disease, traumatic brain injury, and autistic spectrum disorders.
[19] The extended release amantadine formulation is commonly used to treat dyskinesias in people receiving levodopa therapy for Parkinson's disease.
[19] A 2003 Cochrane review had concluded evidence was insufficient to prove the safety or efficacy of amantadine to treat dyskinesia.
[medical citation needed] The CDC recommends against amantadine and rimantadine to treat influenza A infections.
[medical citation needed] A 2014 Cochrane review did not find evidence for efficacy or safety of amantadine used for the prevention or treatment of influenza A.
[22] A 2007 Cochrane literature review concluded that no overall evidence supports the use of amantadine in treating fatigue in patients with multiple sclerosis (MS).
[27] Consensus guidelines from the German Multiple Sclerosis Society (GMSS) in 2006 state that amantadine produces moderate improvement in subjective fatigue, problem solving, memory, and concentration.
Amantadine has been shown to increase the rate of emergence from a MCS, defined by consistent demonstration of interactive communication and functional objective use.
Some case reports also show improved functional recovery with amantadine treatment occurring years after the initial brain injury.
[33] Nonetheless, amantadine-induced acceleration of recovery reduces the burden of disability, lessens health-care costs, and minimizes psychosocial stressors in patients.
[citation needed] Amantadine is contraindicated in persons with end-stage kidney disease,[4] as the drug is renally cleared.
[9] Rare severe adverse effects include neuroleptic malignant syndrome, depression, convulsions, psychosis, and suicidal ideation.
[4] Rare cases of skin rashes, such as Stevens–Johnson syndrome and livedo reticularis have also been reported in patients treated with amantadine.
[38][39] Amantadine inhibits the kidney's active-transport removal and transfer of creatinine from blood to urine, which normally occurs in the proximal tubules of the nephrons.
[44] The effects of amantadine in Parkinson's disease were originally assumed to be anticholinergic or dopaminergic, but the situation soon proved more complicated than this.
[11][12][50] Amantadine shows amphetamine-like psychostimulant effects (e.g., stimulation of locomotor activity) in animals at sufficiently high doses.
[55] Amantadine and rimantadine function in a mechanistically identical fashion, entering the barrel of the tetrameric M2 channel and blocking pore function—i.e., proton translocation.
[57] Amantadine is the organic compound 1-adamantylamine or 1-aminoadamantane, which consists of an adamantane backbone with an amino group substituted at one of the four tertiary carbons.
[60] Rimantadine is a closely related adamantane derivative with similar biological properties;[61] both target the M2 proton channel of influenza A virus.
A majority of the amantadine-resistant H3N2 isolates (98.2%) was found to contain an S31N mutation in the M2 transmembrane domain that confers resistance to amantadine.
[68] An incidental finding in 1969 prompted investigations about amantadine's effectiveness for treating symptoms of Parkinson's disease.
[16] A woman with Parkinson's disease was prescribed amantadine to treat her influenza infection and reported her cogwheel rigidity and tremors improved.
Seven of them showed improvement, which was convincing evidence for the need of a clinical trial, which included 163 patients with Parkinson's disease; 66% experienced subjective or objective reduction of symptoms with a maximum daily dose of 200 mg.[16][69] Additional studies followed patients for greater lengths of time and in different combinations of neurological drugs.
[10][16] In 2017, the U.S. FDA approved the use of amantadine in an extended-release formulation for the treatment of dyskinesia, an adverse effect of levodopa in people with Parkinson's disease.
[75] It is a weak NMDA receptor antagonist and is reported to produce dissociative and phencyclidine-like effects in animals and humans at sufficiently high doses.
[67] Increasing incidence of oseltamivir resistance in circulating influenza strains (e.g., H1N1) exists, highlighting the need for new anti-influenza therapies.
[79] In September 2015, the U.S. FDA announced the recall of Dingo Chip Twists "Chicken in the Middle" dog treats because the product has the potential to be contaminated with amantadine.
[85] A 2021 retrospective study showed that amantadine may serve as an effective adjunct to stimulants for ADHD-related symptoms and appears to be a safer alternative to second- or third-generation antipsychotics.