Amitraz
[3] Its effectiveness is traced back on alpha-adrenergic agonist activity, interaction with octopamine receptors of the central nervous system and inhibition of monoamine oxidases and prostaglandin synthesis.
[1] It is also widely used in the beekeeping industry as a control for the Varroa destructor mite, although there are recent reports of resistance (driven by overuse and off label use).
Therefore, amitraz is available in many different forms, such as a wettable powder, an emulsifiable concentrate, a soluble concentrate/liquid, and an impregnated collar (for dogs).
[1] To apply amitraz, various techniques can be used such as an airblast and concentrate spray to pears or by ground boom and aircraft to cotton.
[8] Territorial differences in amitraz use depend on the species of mites that infest the crops/trees/etc., the local practice, and the number and size of the pear trees.
[6] Besides its application as pesticide on plants, amitraz is also used as an animal ectoparasiticide on cattle, goats, sheep, pigs and dogs.
[9] It achieves special efficiency against mites (first of all Demodex canis), but it also works against lice, flies, and all development stages of ticks.
In some countries amitraz emulsions are also applied to treat demodicosis of cats or dogs, an exceeding infestation of mites of the family Demodicidae.
[9][10] For the treatment of cattle, sheep, goats and pigs amitraz is available as spray- or wash-solution, to treat or prevent infestations by mites, lice, flies and ticks.
[12] Accidental exposure of men to greater amounts of amitraz can lead to death due to respiratory failure, mainly after oral uptake or inhalation.
[3] Since its discovery by Boots Co. in 1969 three main synthesis routes for amitraz has been developed, which stand out in terms of facility and generality.
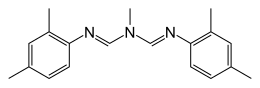
[7] Route 1: 2,4-Xylidine + triethyl orthoformate + methylamine (imine formation/amine formation): [17] One of the first amitraz-manufacturing plants used this reaction scheme (figure 1).
These last steps need to be carried out by instructed personnel, who wear full protective clothing with a positive-pressure breathing apparatus.
This reaction is catalyzed by the presence of acid halides, such as POCl3, SOCl2, COCl2, or an arylsulfonylhalide, as p-toluene sulfonyl chloride (figure 2).
[7][18] This yields an intermediate, which reacts further as its catalyzed by p-toluene acid to N, N'-[(methylimino) dimethylidyne] di-2,4-xylidine (amitraz).
Since amitraz most common use is as a pesticide, it is important to consider that between animals and plants often different pathways for biotransformation occur.
[21] As illustrated in figure 3 the first step is a hydrolysis reaction to N-methyl-N'-(2,4-xylyl)-formamidine, which already can be excreted in the urine but is still pharmacological active.
Therefore, amitraz exposure to humans occurs mainly through inhalation or dermal contact with the compound during its use or production.
[13] The toxic effects to humans following on amitraz-uptake include loss of consciousness, vomiting, respiratory failure, miosis, hypothermia, bradycardia, hyperglycemia and central nervous system depression.
[4] The pharmacological activity of amitraz includes different mechanisms of action leading to toxic effects in humans as well as in animals.
[4] Animal studies revealed that damages due to amitraz poisoning can be recovered even after exposure to a potentially lethal dose.
[24] Xylene present in amitraz formulations additionally induces central nervous system depression.
A mutation can lead to a working version of the octopamine receptor but with an altered pesticide target side.
[29] However, in vivo it has been observed that only at high doses of amitraz or its main metabolite N-2,4-dimethylphenyl-N-methyl-formamide monoamine oxidase inhibition occurs.
[13] In dogs it has been observed that after administration of such a dose an increase in plasma glucose and suppression of insulin occurs.
[13] Like other formamidines amitraz inhibits the synthesis of prostaglandin E2 from arachidonic acid by bovine seminal vesicle microsomes.
[30] In a dose of 5 to 80 mg/kg body weight, given intraperitoneally to rats, amitraz reduces yeast-induced fever and antagonizes the carrageenin-induced swelling of the hind paw.
[30] Some of the physiological effects of amitraz probably go back to this aspirin-like activity and occur due to inhibition of prostaglandin synthesis.