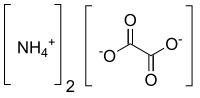
Ammonium oxalate
It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4).
It is a colorless or white salt under standard conditions and is odorless and non-volatile.
Oxammite is a natural mineral form of ammonium oxalate.
[5] Ammonium oxalate is used as an analytical reagent and general reducing agent.
[citation needed] Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron(II)-bearing minerals (such as magnetite) and organic matter.