Amphiphile
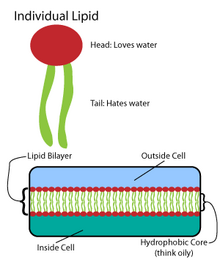
In chemistry, an amphiphile (from Greek αμφις (amphis) 'both' and φιλíα (philia) 'love, friendship'), or amphipath, is a chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving, nonpolar) properties.
[citation needed] Amphiphilic compounds have lipophilic (typically hydrocarbon) structures and hydrophilic polar functional groups (either ionic or uncharged).
[citation needed] As a result of having both lipophilic and hydrophilic portions, some amphiphilic compounds may dissolve in water and to some extent in non-polar organic solvents.
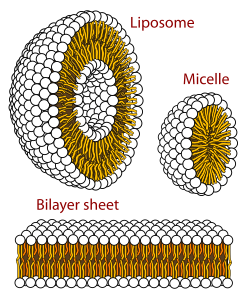
The two layers then stack such that their lyphphilic chains touch on the inside and their polar groups are outside facing the surrounding aqueous media.
Some typical members of this group are: sodium dodecyl sulfate (anionic), benzalkonium chloride (cationic), cocamidopropyl betaine (zwitterionic), and 1-octanol (long-chain alcohol, non-ionic).
Soap mixed with water (polar, hydrophilic) is useful for cleaning oils and fats (non-polar, lipophilic) from kitchenware, dishes, skin, clothing, etc.