Hydrophobe
Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general.
[citation needed] The hydrophobic interaction is mostly an entropic effect originating from the disruption of the highly dynamic hydrogen bonds between molecules of liquid water by the nonpolar solute, causing the water to form a clathrate-like structure around the non-polar molecules.
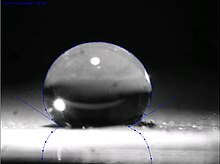
[citation needed] Superhydrophobic surfaces, such as the leaves of the lotus plant, are those that are extremely difficult to wet.
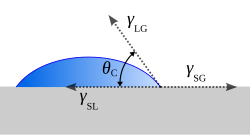
[7] In 1805, Thomas Young defined the contact angle θ by analyzing the forces acting on a fluid droplet resting on a solid surface surrounded by a gas.
[citation needed] We can predict whether the Wenzel or Cassie–Baxter state should exist by calculating the new contact angle with both equations.
By a minimization of free energy argument, the relation that predicted the smaller new contact angle is the state most likely to exist.
[citation needed] Dettre and Johnson discovered in 1964 that the superhydrophobic lotus effect phenomenon was related to rough hydrophobic surfaces, and they developed a theoretical model based on experiments with glass beads coated with paraffin or TFE telomer.
[17] Perfluoroalkyl, perfluoropolyether, and RF plasma -formed superhydrophobic materials were developed, used for electrowetting and commercialized for bio-medical applications between 1986 and 1995.
[23] In recent research, superhydrophobicity has been reported by allowing alkylketene dimer (AKD) to solidify into a nanostructured fractal surface.
[31] Many hydrophobic materials found in nature rely on Cassie's law and are biphasic on the submicrometer level with one component air.
[citation needed] One study presents a vanadium pentoxide surface that switches reversibly between superhydrophobicity and superhydrophilicity under the influence of UV radiation.
[33] According to the study, any surface can be modified to this effect by application of a suspension of rose-like V2O5 particles, for instance with an inkjet printer.
That is to say, the presence of molecular species (usually organic) or structural features results in high contact angles of water.
[34] The intrinsic hydrophobicity of rare earth oxides depends on surface orientation and oxygen vacancy levels, and is naturally more robust than coatings or surface treatments, having potential applications in condensers and catalysts that can operate at high temperatures or corrosive environments.
[citation needed] Active recent research on superhydrophobic materials might eventually lead to more industrial applications.
Patterned superhydrophobic surfaces also have promise for lab-on-a-chip microfluidic devices and can drastically improve surface-based bioanalysis.