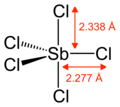
Antimony pentachloride
It is a colourless oil, but typical samples are yellowish due to dissolved chlorine.
Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
[6] This compounds reacts with water to form antimony pentoxide and hydrochloric acid:[7] The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O.
[10] For example aromatic ethers are oxidized to their radical cations according to the following stoichiometry:[11] Antimony pentachloride is used as a polymerization catalyst and for the chlorination of organic compounds.
It is a chlorinating agent and, in the presence of moisture, it releases hydrogen chloride gas.