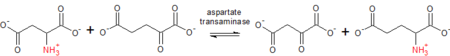Aspartate transaminase
[1][2][3] AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism.
AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder.
L-Aspartate (Asp) + α-ketoglutarate ↔ oxaloacetate + L-glutamate (Glu) As a prototypical transaminase, AST relies on PLP (Vitamin B6) as a cofactor to transfer the amino group from aspartate or glutamate to the corresponding ketoacid.
In humans:[citation needed] These isoenzymes are thought to have evolved from a common ancestral AST via gene duplication, and they share a sequence homology of approximately 45%.
[10] X-ray crystallography studies have been performed to determine the structure of aspartate transaminase from various sources, including chicken mitochondria,[11] pig heart cytosol,[12] and E.
The large domain, which includes residues 48-325, binds the PLP cofactor via an aldimine linkage to the ε-amino group of Lys258.
The small domain consists of residues 15-47 and 326-410 and represents a flexible region that shifts the enzyme from an "open" to a "closed" conformation upon substrate binding.
In the first half-reaction, amino acid 1 (e.g., L-Asp) reacts with the enzyme-PLP complex to generate ketoacid 1 (oxaloacetate) and the modified enzyme-PMP.
In the second half-reaction, ketoacid 2 (α-ketoglutarate) reacts with enzyme-PMP to produce amino acid 2 (L-Glu), regenerating the original enzyme-PLP in the process.