Bafilomycin
Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.
[8] Bafilomycin A1 specifically targets the vacuolar-type H+ -ATPase (V-ATPase) enzyme, a membrane-spanning proton pump that acidifies either the extracellular environment or intracellular organelles such as the lysosome of animal cells or the vacuole of plants and fungi.
[2][10] Bafilomycin A1 serves as an important tool compound in many in vitro research applications; however, its clinical use is limited by a substantial toxicity profile.
Bafilomycin C1 was found to have activity against Caenorhabditis elegans, ticks, and tapeworms, in addition to stimulating the release of γ-aminobutyruc acid (GABA) from rat synaptosomes.
Independently, bafilomycin A1 and other derivatives were isolated from S. griseus and shown to have antibiotic activity against some yeast, Gram-positive bacteria and fungi.
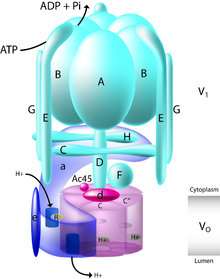
[14] Structurally, V-ATPase consists of 13 distinct subunits that together make up the membrane spanning Vo and cytosolic V1 domains of the enzyme.
Further narrowing bafilomycin's interaction site, they found that specific addition of just Vo subunit a could restore function.
These data suggested that the bafilomycin binding site was on the outer surface of the Vo domain, at the interface between two c subunits.
[16][17] This binding site has recently been described in high resolution by two groups that used cryo electron microscopy to obtain structures of the V-ATPase bound to bafilomycin.
[18][19] Overall, bafilomycin binds with nanomolar efficiency to the Vo c subunit of the V-ATPase complex and inhibits proton translocation.
[3] When at the plasma membrane, V-ATPase function is critical in the acidification of the extracellular environment, which is seen with osteoclasts and epididymal clear cells.
When present at the plasma membrane in renal epithelial intercalated cells, V-ATPase is important for acid secretion, which contributes to the acidification of urine.
[12] This was initially found in a paper by Yamamoto, et al. in which the authors used bafilomycin A1 to treat rat hepatoma H-4-II-E cells.
This has been confirmed by other studies, particularly two that found decreased colocalization of mitochondria and lysosomes by fluorescence microscopy following a 12-24 hour treatment with 100 or 400 nM Bafilomycin.
[20] Additionally, in some cell lines it has been found to disrupt the electrochemical gradient of the mitochondria and induce the release of cytochrome c, which is an initiator of apoptosis.
Ascending concentrations of bafilomycin were found to linearly increase the amount of K+ that traversed the mitochondrial membrane, confirming it acts as an ionophore.
These effects suggest that inhibition of V-ATPase with bafilomycin can induce a cellular stress response, including autophagy and eventual apoptosis.
[3][24] In vivo bafilomycin reduced average tumor volume in MCF-7 and MDA-MB-231 xenograft mouse models by 50% and did not show toxic effects at a dosing of 1 mg/kg.
[3] V-ATPase dysregulation is thought to play a role in resistance to cancer therapies, as aberrant acidification of the extracellular environment can protonate chemotherapeutics, preventing their entry into the cell.
[3] Bafilomycins have been shown to inhibit plasma membrane ATPase (P-ATPase) as well as the ATP-binding cassette (ABC) transporters.
This proton pump has a role in maintaining the intracellular pH of the infected red blood cell and facilitating the uptake of small metabolites at equilibrium.
[32] The inflammatory myopathy Inclusion Body Myositis (IBM) is relatively common in patients over 50 years of age and involves over activation of autophagic flux.
Treatment with bafilomycin can prevent the acidification of lysosomes and therefore autophagy, decreasing the number of antigenic peptides digested and displayed to the immune system.
Trapping of the cationic compound also draws water into the lysosome through an osmotic effect, which can sometimes lead to vacuolization seen in in vitro cultured cells.
The lipophilic agent xylometazoline, an alpha-adrenoreceptor agonist, displayed an increased effect when administered after bafilomycin treatment.
Without pre-treatment with bafilomycin, the functional V-ATPase causes the lysosome to become a reservoir for xylometazoline, slowing its effect on contractility.
[21] As a lysosomotropic drug, chloroquine typically accumulates in the lysosome disrupting their degradative function, inhibiting autophagy, and inducing apoptosis through Bax-dependent mechanisms.
The exact mechanism of this protection is unknown, although it is hypothesized to lie downstream of autophagosome-lysosome fusion yet upstream of Bax induction of apoptosis.
[12] Bafilomycin has been shown to potentiate the effect of taxol in decreasing Matrix Metalloprotease (MMP) levels by depressing Bcl-xL's mitochondrial protective role.