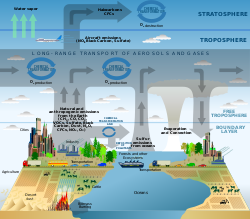
Atmospheric chemistry
This multidisciplinary approach of research draws on environmental chemistry, physics, meteorology, computer modeling, oceanography, geology and volcanology, climatology and other disciplines to understand both natural and human-induced changes in atmospheric composition.
These trace gasses include compounds such as CFCs/HCFCs which are particularly damaging to the ozone layer, and H2S which has a characteristic foul odor of rotten eggs and can be smelt in concentrations as low as 0.47 ppb.
Further studies on ozone issues led to the 1995 Nobel Prize in Chemistry award shared between Paul Crutzen, Mario Molina and Frank Sherwood Rowland.
The changing climate and the recovery of the ozone hole and the interaction of the composition of the atmosphere with the oceans and terrestrial ecosystems are examples of the interdependent relationships between Earth's systems.
Astrochemists analyze the atmospheric compositions of our solar system and exoplanets to determine the formation of astronomical objects and find habitual conditions for Earth-like life.
Observatories such as the Mauna Loa and mobile platforms such as aircraft ships and balloons (e.g. the UK's Facility for Airborne Atmospheric Measurements) study chemical compositions and weather dynamics.
Observations of atmospheric composition are increasingly made by satellites by passive and active remote sensing with important instruments such as GOME and MOPITT giving a global picture of air pollution and chemistry.
Some surface based instruments e.g. LIDAR can provide concentration profiles of chemical compounds and aerosols but are still restricted in the horizontal region they can cover.
Experiments are performed in controlled environments, such as aerosol chambers, that allow for the individual evaluation of specific chemical reactions or the assessment of properties of a particular atmospheric constituent.
[15] A closely related subdiscipline is atmospheric photochemistry, which quantifies the rate that molecules are split apart by sunlight, determines the resulting products, and obtains thermodynamic data such as Henry's law coefficients.
Commonly used instruments to measure aerosols include ambient and particulate air samplers, scanning mobility particle sizers, and mass spectrometers.
For example, box modeling is relatively simple and may include hundreds or even thousands of chemical reactions, but they typically use a very crude representation of atmospheric mixed layer.
This approach has gained attention over the past decade as an effective method to interpret large amounts of data generate by models and observations from satellites.
Atmospheric chemistry is a multidisciplinary field with wide-ranging applications that influence environmental policy, human health, technology development, and climate science.
Examples of problems addressed in atmospheric chemistry include acid rain, ozone depletion, photochemical smog, greenhouse gasses and global warming.
Atmospheric chemistry also helps quantify the concentration and persistence of toxic substances in the air, including particulate matter and volatile organic compounds (VOCs), guiding public health measures and exposures assessments.