Nanochemistry
[1] The term "nanochemistry" was first used by Ozin in 1992 as 'the uses of chemical synthesis to reproducibly afford nanomaterials from the atom "up", contrary to the nanoengineering and nanophysics approach that operates from the bulk "down"'.
[2] Nanochemistry focuses on solid-state chemistry that emphasizes synthesis of building blocks that are dependent on size, surface, shape, and defect properties, rather than the actual production of matter.
Silica, gold, polydimethylsiloxane, cadmium selenide, iron oxide, and carbon are materials that show its transformative power.
Nanochemistry can make the most effective contrast agent of MRI out of iron oxide (rust) which can detect cancers and kill them at their initial stages.
[6] Nano-construct synthesis leads to the self-assembly of the building blocks into functional structures that may be useful for electronic, photonic, medical, or bioanalytical problems.
By the early 1940’s, precipitated and fumed silica nanoparticles were being manufactured and sold in USA and Germany as substitutes for ultrafine carbon black for rubber reinforcements.
[8] Over the past two decades, iron oxide nanoparticles for biomedical use had increased dramatically, largely due to its ability of non-invasive imaging, targeting and triggering drug release, or cancer therapy.
Stem or immune cell could be marked with iron oxide nanoparticles to be detected by Magnetic resonance imaging (MRI).
Mesoporous silica nanoparticles (MSN) have increased in research popularity due to their large surface area and flexibility for various individual modifications while maintaining high-resolution performance under imaging techniques.
[11] The two-photon activated photo-transducer (2-NPT) uses near infrared wavelengths of light to induce the breaking of a disulfide bond to release the cargo.
[12] Recently, nanodiamonds have demonstrated potential in drug delivery due to non-toxicity, spontaneous absorption through the skin, and the ability to enter the blood–brain barrier.
[15] Also, carbon nanotubes can be designed to match the size of small drug and endocitozed by a target cell, hence becoming a delivery agent.
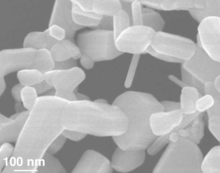
[23] Scientists have devised a large number of nanowire compositions with controlled length, diameter, doping, and surface structure by using vapor and solution phase strategies.
In addition, their efficiency in electron transport which is due to the quantum confinement effect, makes their electrical properties be influenced by minor perturbation.
They have concluded that using short wavelength nanolasers has applications in different fields such as optical computing, information storage, and microanalysis.
Nanodiamonds can be synthesized by employing nanoscale carbonaceous seeds created in a single step by using a mask-free electron beam-induced position technique to add amine groups.
[28] This process affords a high yield that relies on covalent bonding between the amine and carboxyl functional groups on amorphous carbon and nanodiamond surfaces in the presence of EDC.
[29] The invention received a US patent in 2008 States7326837 B2 United States 7326837 B2, Chau-Chung Han; Huan-Cheng Chang & Shen-Chung Lee et al., "Clinical applications of crystalline diamond particles", issued February 5, 2008, assigned to Academia Sinica, Taipei (TW) , and a subsequent patent in 2012 States8168413 B2 United States 8168413 B2, Huan-Cheng Chang; Wunshian Fann & Chau-Chung Han, "Luminescent Diamond Particles", issued May 1, 2012, assigned to Academia Sinica, Taipei (TW) .
[31] Nanodiamonds can self-assemble and a wide range of small molecules, proteins antibodies, therapeutics, and nucleic acids can bind to its surface allowing for drug delivery, protein-mimicking, and surgical implants.
Nanodiamonds are capable of biocompatibility, the ability to carry a broad range of therapeutics, dispersibility in water and scalability, and the potential for targeted therapy all properties needed for a drug delivery platform.
The small size, stable core, rich surface chemistry, ability to self-assemble, and low cytotoxicity of nanodiamonds have led to suggestions that they could be used to mimic globular proteins.
[33] Monodisperse, nanometer-size clusters (also known as nanoclusters) are synthetically grown crystals whose size and structure influence their properties through the effects of quantum confinement.
[34] Dielectric properties of nanoclusters are also a subject of interest due to their possible applications in catalysis, photocatalysis, micro capacitors, microelectronics, and nonlinear optics.
[35] The idea of nanothermodynamics was initially proposed by T. L. Hill in 1960, theorizing the differences between differential and integral forms of properties due to small sizes.
[40] The technologies include nanowires, a new class of quasi-one-dimensional materials that have demonstrated superior electrical, optical, mechanical, and thermal properties and can be used potentially as biological sensors.
[41] Shimon Weiss, a professor at the University of California, Los Angeles, is known for his research of fluorescent semiconductor nanocrystals, a subclass of quantum dots, for biological labeling.