Autoclave
Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis.
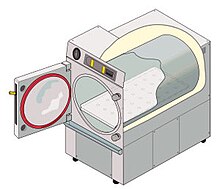
[5] Sterilization autoclaves are widely used in microbiology and mycology, medicine and prosthetics fabrication, tattooing and body piercing, and funerary practice.
They vary in size and function depending on the media to be sterilized and are sometimes called retort in the chemical and food industries.
Machines in this category largely operate under the same principles as conventional autoclaves in that they are able to neutralize (but not eliminate) potentially infectious agents by using pressurized steam and superheated water.
[8] Autoclaves are also widely used to cure composites, especially for melding multiple layers without any voids that would decrease material strength, and in the vulcanization of rubber.
[13] However, prions, such as those associated with Creutzfeldt–Jakob disease, and some toxins released by certain bacteria, such as Cereulide, may not be destroyed by autoclaving at the typical 134 °C for three minutes or 121 °C for 15 minutes and instead should be immersed in sodium hydroxide (1M NaOH) and heated in a gravity displacement autoclave at 121 °C for 30 min, cleaned, rinsed in water and subjected to routine sterilization.
Because they are optimized for continuous hospital use, they favor rectangular designs, require demanding maintenance regimens, and are costly to operate.
(A properly calibrated medical-grade autoclave uses thousands of gallons of water each day, independent of task, with correspondingly high electric power consumption.)
In 2016, the Office of Sustainability at the University of California, Riverside (UCR) conducted a study of autoclave efficiency in their genomics and entomology research labs, tracking several units' power and water consumption.
They monitored side-by-side autoclaves in a facility running cycles indicative of the most common load and sterilization tasks used by their researchers campuswide.
The author also noted anecdotal reports of electrical utility and maintenance cost savings associated with non-jacketed autoclaves.
[18] Research autoclaves display a wide range of designs and sizes, and are frequently tailored to their use and load type.
A 2017 study performed by the Johns Hopkins Hospital biocontainment unit tested the ability of pass-through autoclaves to decontaminate loads of simulated biomedical waste when run on the factory default setting.
It is designed specifically to prove that the process achieved full temperature and time required for a normal minimum cycle of 134 °C for 3.5–4 minutes.
If the autoclave does not reach the right temperature, the spores will germinate when incubated and their metabolism will change the color of a pH-sensitive chemical.
Some physical indicators consist of an alloy designed to melt only after being subjected to a given temperature for the relevant holding time.