BRCA1
[15] The BRCA1 protein associates with RNA polymerase II, and through the C-terminal domain, also interacts with histone deacetylase complexes.
[16] Methods to test for the likelihood of a patient with mutations in BRCA1 and BRCA2 developing cancer were covered by patents owned or controlled by Myriad Genetics.
[21] After an international race to refine the precise location of BRCA1,[22] the gene was cloned in 1994 by scientists at University of Utah, National Institute of Environmental Health Sciences (NIEHS) and Myriad Genetics.
[28] This motif contains a short anti-parallel beta-sheet, two zinc-binding loops and a central alpha helix in a small domain.
The BARD1/BRCA1 interaction is disrupted by tumorigenic amino acid substitutions in BRCA1, implying that the formation of a stable complex between these proteins may be an essential aspect of BRCA1 tumor suppression.
BRCA1 polypeptides, in particular, Lys-48-linked polyubiquitin chains are dispersed throughout the resting cell nucleus, but at the start of DNA replication, they gather in restrained groups that also contain BRCA2 and BARD1.
[31] The C-terminal BRCT region of the BRCA1 protein is essential for repair of DNA, transcription regulation and tumor-suppressor function.
[33] In BRCA1 the dual tandem repeat BRCT domains are arranged in a head-to-tail-fashion in the three-dimensional structure, burying 1600 Å of hydrophobic, solvent-accessible surface area in the interface.
A missense mutation at the interface of these two proteins can perturb the cell cycle, resulting a greater risk of developing cancer.
[35] FA-S is almost always a lethal condition in utero; only a handful cases of biallelic BRCA1 mutations have been reported in literature despite the high carrier frequencies in the Ashkenazim, and none since 2013.
[37] These breaks can be caused by natural radiation or other exposures, but also occur when chromosomes exchange genetic material (homologous recombination, e.g., "crossing over" during meiosis).
After ionizing radiation, VCP is recruited to DNA lesions and cooperates with the ubiquitin ligase RNF8 to orchestrate assembly of signaling complexes for efficient DSB repair.
[44] This may explain a role for BRCA1 to promote lower fidelity DNA repair by non-homologous end joining (NHEJ).
[16][46] Formaldehyde and acetaldehyde are common environmental sources of DNA cross links that often require repairs mediated by BRCA1 containing pathways.
[57] Newer methods have also been recently proposed: heteroduplex analysis (HDA) by multi-capillary electrophoresis or also dedicated oligonucleotides array based on comparative genomic hybridization (array-CGH).
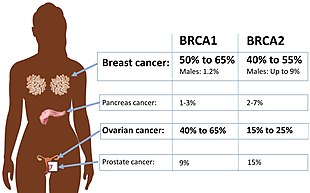
[11] Women who have inherited a defective BRCA1 or BRCA2 gene are at a greatly elevated risk to develop breast and ovarian cancer.
An innate genomic deficit in a tumor suppressor gene impairs normal responses and exacerbates the susceptibility to disease in organ targets.
[62] It has long been noted that loss of BRCA1 activity, either by germ-line mutations or by down-regulation of gene expression, leads to tumor formation in specific target tissues.
[63] Reduced expression of BRCA1 is tumorigenic because it plays an important role in the repair of DNA damages, especially double-strand breaks, by the potentially error-free pathway of homologous recombination.
In particular this deficiency initiates a cascade of molecular events that sculpt the evolution of high-grade serous ovarian cancer and dictate its response to therapy.
Three mutations in BRCA1 have been reported to account for the majority of Ashkenazi Jewish patients with inherited BRCA1-related breast and/or ovarian cancer: 185delAG, 188del11 and 5382insC in the BRCA1 gene.
As ovarian reserve and fertility decline with age, there is also a parallel increase in pregnancy failure and meiotic errors, resulting in chromosomally abnormal conceptions.
Persons with NSCLC are often treated with therapeutic platinum compounds (e.g. cisplatin, carboplatin or oxaliplatin) that cause inter-strand cross-links in DNA.
Among individuals with NSCLC, low expression of BRCA1 in the primary tumor correlated with improved survival after platinum-containing chemotherapy.
High BRCA1 may protect cancer cells by acting in a pathway that removes the damages in DNA introduced by the platinum drugs.
[125] A patent application for the isolated BRCA1 gene and cancer promoting mutations discussed above, as well as methods to diagnose the likelihood of getting breast cancer, was filed by the University of Utah, National Institute of Environmental Health Sciences (NIEHS) and Myriad Genetics in 1994;[17] over the next year, Myriad, (in collaboration with investigators at Endo Recherche, Inc., HSC Research & Development Limited Partnership, and University of Pennsylvania), isolated and sequenced the BRCA2 gene and identified key mutations, and the first BRCA2 patent was filed in the U.S. by Myriad and other institutions in 1995.
In Australia and the UK, Myriad's licensee permitted use by health systems but announced a change of plans in August 2008.
In effect, the United States is the only jurisdiction where Myriad's strong patent position has conferred sole-provider status.
[131] The Federal Court of Australia came to the opposite conclusion, upholding the validity of an Australian Myriad Genetics patent over the BRCA1 gene in February 2013.
[133] Yvonne D'Arcy won her case against US-based biotech company Myriad Genetics in the High Court of Australia.