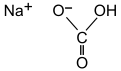
Sodium bicarbonate
[citation needed] Heat can also by itself cause sodium bicarbonate to act as a raising agent in baking because of thermal decomposition, releasing carbon dioxide at temperatures above 80 °C (180 °F), as follows:[16] When used this way on its own, without the presence of an acidic component (whether in the batter or by the use of a baking powder containing acid), only half the available CO2 is released (one CO2 molecule is formed for every two equivalents of NaHCO3).
[24] Sodium bicarbonate also delays combustion reactions through the release of carbon dioxide and water, both of which are flame retardants, when heated.
[27] As baking soda will absorb musty smells, it has become a reliable method for used booksellers when making books less malodorous.
The alkaline nature of sodium bicarbonate makes it the only dry chemical agent, besides Purple-K, that was used in large-scale fire suppression systems installed in commercial kitchens.
[35] Studies conducted mostly in males have shown that sodium bicarbonate is most effective in enhancing performance in short-term, high-intensity activities.
[38][39] Its reaction with stomach acid produces salt, water, and carbon dioxide: A mixture of sodium bicarbonate and polyethylene glycol such as PegLyte,[40] dissolved in water and taken orally, is an effective gastrointestinal lavage preparation and laxative prior to gastrointestinal surgery, gastroscopy, etc.
[41] In cases of respiratory acidosis, the infused bicarbonate ion drives the carbonic acid/bicarbonate buffer of plasma to the left, and thus raises the pH.
[48] Sodium bicarbonate can be added to local anaesthetics, to speed up the onset of their effects and make their injection less painful.
This makes the tablet disintegrate, leaving the medication suspended and/or dissolved in the water together with the resulting salt (in this example, sodium tartrate).
[56] It works as a mechanical cleanser on the teeth and gums, neutralizes the production of acid in the mouth, and also acts as an antiseptic to help prevent infections.
[59][60] Sodium bicarbonate may be used as a buffering agent, combined with table salt, when creating a solution for nasal irrigation.
This is done by adding a teaspoon of sodium bicarbonate to cool water that was recently boiled followed by gentle scrubbing of the eyelash base with a cotton swab dipped in the solution.
As a blasting medium, sodium bicarbonate is used to remove surface contamination from softer and less resilient substrates such as aluminium, copper, or timber that could be damaged by silica sand abrasive media.
[65] A manufacturer recommends a paste made from baking soda with minimal water as a gentle scouring powder.
[67] Sodium bicarbonate attacks the thin protective oxide layer that forms on aluminium, making it unsuitable for cleaning this metal.
[68][69] Baking soda is commonly added to washing machines as a replacement for water softener and to remove odors from clothes.
When diluted with warm water, it is also almost as effective in removing heavy tea and coffee stains from cups as sodium hydroxide.
Clothing can become contaminated with toxic dust of depleted uranium (DU), which is very dense, hence it is used for counterweights in a civilian context and in armour-piercing projectiles.
[72] This idea was promoted by the leading U.S. brand of baking soda, Arm & Hammer, in an advertising campaign starting in 1972.
[73] Though this campaign is considered a classic of marketing, leading within a year to more than half of American refrigerators containing a box of baking soda,[74][75] there is little evidence that it is effective in this application.
[78] If this reaction is performed inside of a closed vessel (such as a bottle) with no way for gas to escape, it can cause an explosion if the pressure is high enough.
[83] If kept cool (room temperature) and dry (an airtight container is recommended to keep out moist air), sodium bicarbonate can be kept without a significant amount of decomposition for at least two or three years.
the Lower Egyptian “Natrontal” Wadi El Natrun, where a mixture of sodium carbonate and sodium hydrogen carbonate for the dehydration of mummies was used [88]) from Greek nítron (νίτρον) (Herodotus; Attic lítron (λίτρον)), which can be traced back to ancient Egyptian ntr.
The Greek nítron (soda, saltpeter) was also used in Latin (sal) nitrum and in German Salniter (the source of Nitrogen, Nitrat etc.).
"I want to say, for the benefit of those who are making this investigation," he reported, "that I was told by a judge of a superior court in the mountain country of North Carolina they have discovered a remedy for this disease."
[citation needed] Although of no practical value, NaHCO3 may be obtained by the reaction of carbon dioxide with an aqueous solution of sodium hydroxide:[citation needed] Naturally occurring deposits of nahcolite (NaHCO3) are found in the Eocene-age (55.8–33.9 Mya) Green River Formation, Piceance Basin in Colorado.
"[99] In the Joseph L. Mankewicz classic All About Eve, the Max Fabian character (Gregory Ratoff) has an extended scene with Margo Channing (Bette Davis) in which, suffering from heartburn, he requests and then drinks bicarbonate of soda, eliciting a prominent burp.