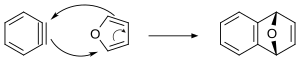Aryne
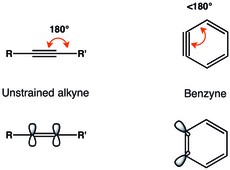
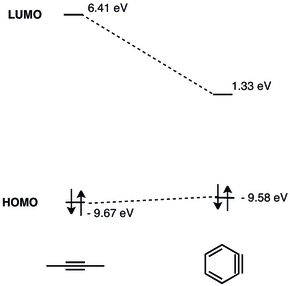
In organic chemistry, arynes[1] and benzynes[2] are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents.
Early routes to benzyne involved dehydrohalogenation of aryl halides: Such reactions require strong base and high temperatures.
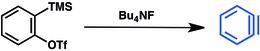
[15] Fluoride displacement of the trimethylsilyl group induces elimination of triflate and release of benzyne: A hexadehydro Diels-Alder reaction (HDDA) involves cycloaddition of 1,3-diyne and alkyne.
[16] N-amination of 1H-benzotriazole with hydroxylamine-O-sulfonic acid generates an intermediate which can be oxidised to benzyne in almost quantitative yield with lead(IV) acetate.
Their reactivity can be classified in three main classes: (1) nucleophilic additions, (2) pericyclic reactions, and (3) bond-insertion.
Upon treatment with basic nucleophiles, aryl halides deprotonate alpha to the leaving group, resulting in dehydrohalogenation.
Aryl bromides and iodides, on the other hand, generally appear to undergo elimination by a concerted syn-coplanar E2 mechanism.
[20] "Aryne coupling" reactions allow for generation of biphenyl compounds which are valuable in pharmaceutical industry, agriculture and as ligands in many metal-catalyzed transformations.
Using copper(I) cyanide as the initiator to add to the first aryne yielded polymers containing up to about 100 arene units.
[27] Tetrabromobenzene can react with butyllithium and furan to form a tetrahydroanthracene[28] [4+2] cycloadditions of arynes have been commonly applied to natural product total synthesis.
Due to electrophilic nature of benzyne, alkenes bearing electron-donating substituents work best for this reaction.
[30] Due to significant byproduct formation, aryne [2+2] chemistry is rarely utilized in natural product total synthesis.
In 1982, Stevens and co-workers reported a synthesis of taxodione that utilized [2+2] cycloaddition between an aryne and a ketene acetal.
[34][35] A 1,2- to 1,3-didehydrobenzene conversion has been postulated to occur in the pyrolysis (900 °C) of the phenyl substituted aryne precursors[34] as shown below.
Chen proposed the use of 1,4-didehydrobenzene analogues that have large singlet-triplet energy gaps to enhance selectivity of enediyne drug candidates.
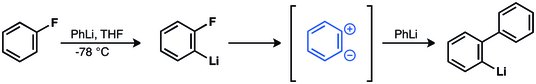
[40] Wittig et al. invoked zwitterionic intermediate in the reaction of fluorobenzene and phenyllithium to give biphenyl.
[44] John D. Roberts et al. showed that the reaction of chlorobenzene-1-14C and potassium amide gave equal amounts of aniline with 14C incorporation at C-1 and C-2.
[54] Their approach employed an aryne cyclization to close the final ring of the natural product.
Despite their potential utility, examples of multicomponent aryne reactions in natural product synthesis are scarce.