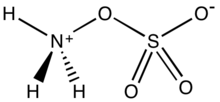
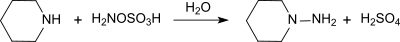
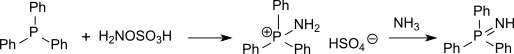
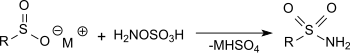Hydroxylamine-O-sulfonic acid
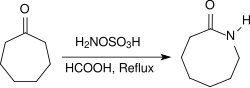
It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.
[6] The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid[3] by a method first published in 1925[7] and refined for Organic Syntheses.
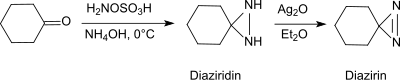
[21] Under basic conditions in the presence of primary amines, hydroxylamine-O-sulfonic acid forms with aldehydes and ketones (e.g. cyclohexanone[22]) diaziridines, which can easily be oxidized to the more stable diazirines.
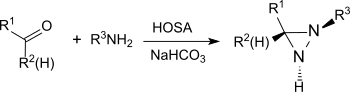
The reaction also provides substituted aziridines from simple aldehydes and ketones with high yield and diastereoselectivity.
[23] 1,2-Benzisoxazole is efficiently produced by nucleophilic attack of hydroxylamine-O-sulfonic acid to the carbonyl group of 2-hydroxybenzaldehyde followed by cyclization.
In a one-pot reaction, N-aryl[3,4-d]pyrazolopyrimidines are obtained in good yields from simple 4,6-dichloropyrimidine-5-carboxaldehyde,[25] which can be used as purine analogs for a wide range of diagnostic and therapeutic applications.