Tin
Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4.
Pewter, which is an alloy of 85–90% tin with the remainder commonly consisting of copper, antimony, bismuth, and sometimes lead and silver, has been used for flatware since the Bronze Age.
[18] Some unverifiable sources also say that, during Napoleon's Russian campaign of 1812, the temperatures became so cold that the tin buttons on the soldiers' uniforms disintegrated over time, contributing to the defeat of the Grande Armée,[19] a persistent legend.
[citation needed] Tin is one of the easiest elements to detect and analyze by NMR spectroscopy, which relies on molecular weight and its chemical shifts are referenced against tetramethyltin (SnMe4).
Current studies are for lead or lead-bismuth reactor coolants because both heavy metals are nearly transparent to fast neutrons, with very low capture cross sections.
The other six isotopes forming 82.7% of natural tin have capture cross sections of 0.3 barns or less, making them effectively transparent to neutrons.
Tin-100 and tin-132 are two of the very few nuclides with a "doubly magic" nucleus which despite being unstable, as they have very uneven neutron–proton ratios, are the endpoints beyond which tin isotopes lighter than tin-100 and heavier than tin-132 are much less stable.
Stannum apparently came from an earlier stāgnum (meaning the same substance),[36] the origin of the Romance and Celtic terms for tin, such as French étain, Spanish estaño, Italian stagno, and Irish stán.
[40] The Meyers Konversations-Lexikon suggests instead that stannum came from Cornish stean, and is evidence that Cornwall in the first centuries AD was the main source of tin.
Cassiterite often accumulates in alluvial channels as placer deposits because it is harder, heavier, and more chemically resistant than the accompanying granite.
Alternatively SnCl4 and Sn combine to stannous chloride by a process called comproportionation:[47] Tin can form many oxides, sulfides, and other chalcogenide derivatives.
Organotin(II) compounds include both stannylenes (formula: R2Sn, as seen for singlet carbenes) and distannylenes (R4Sn2), which are roughly equivalent to alkenes.
In 1984, the Association of Tin Producing Countries was created, with Australia, Bolivia, Indonesia, Malaysia, Nigeria, Thailand, and Zaire as members.
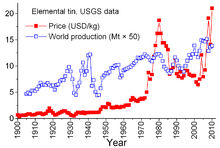
However, the buffer stockpile was not sufficiently large, and during most of those 29 years tin prices rose, sometimes sharply, especially from 1973 through 1980 when rampant inflation plagued many world economies.
[73] Due to factors involved in the 2021 global supply chain crisis, tin prices almost doubled during 2020–21 and have had their largest annual rise in over 30 years.
[76] Purple of Cassius, Pigment Red 109, a hydrous double stannate of gold, was mainly, in terms of painting, restricted to miniatures due to its high cost.
[citation needed] Cerulean blue, a somewhat dull cyan chemically known as cobalt stannate, continues to be an important artists' pigment.
[82] NTP Yellow possesses the highest level of color saturation of these contemporary inorganic mixed metal complex pigments.
Tin in form of ingots provide the raw material necessary for these chemical reactions, ensuring consistent quality and performance.
[106] Punched tin-plated steel, also called pierced tin, is an artisan technique originating in central Europe for creating functional and decorative housewares.
These cabinets had tinplate inserts in the doors and sometimes in the sides, punched out by the homeowner, cabinetmaker, or a tinsmith in varying designs to allow for air circulation while excluding flies.
In the absence of such stabilizers, PVC would rapidly degrade under heat, light, and atmospheric oxygen, resulting in discolored, brittle products.
[123] Because of this persistence and its use as an additive in ship paint, high concentrations of tributyltin have been found in marine sediments located near naval docks.
[125] With the high levels of TBT in the local inshore areas, due to shipping activities, the shellfish had an adverse effect.
[123] Imposex is the imposition of male sexual characteristics on female specimens where they grow a penis and a pallial vas deferens.
[125][126] A high level of TBT can damage mammalian endocrine glands, reproductive and central nervous systems, bone structure and gastrointestinal tract.
In the largest application, stannous chloride is a common reducing agent for the conversion of nitro and oxime groups to amines.
[127] Tin forms several inter-metallic phases with lithium metal, making it a potentially attractive material for battery applications.
Large volumetric expansion of tin upon alloying with lithium and instability of the tin-organic electrolyte interface at low electrochemical potentials are the greatest challenges to employment in commercial cells.
The National Institute for Occupational Safety and Health (NIOSH) determined a recommended exposure limit (REL) of 2 mg/m3 over an 8-hour workday.




2 ) [ 49 ]