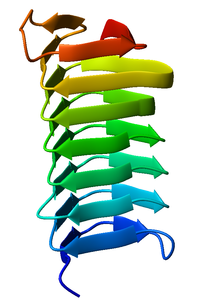
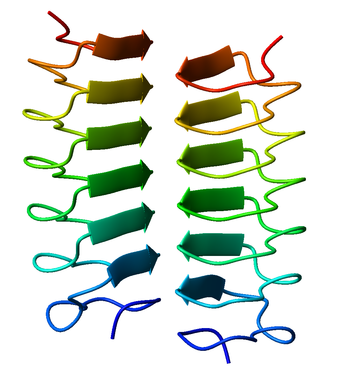
Beta helix
[3][4] The first beta-helix was observed in the enzyme pectate lyase, which contains a seven-turn helix that reaches 34 Å (3.4 nm) long.
The P22 phage tail spike protein, a component of the P22 bacteriophage, has 13 turns and in its assembled homotrimer is 200 Å (20 nm) in length.
Both pectate lyase and P22 tailspike protein contain right-handed helices; left-handed versions have been observed in enzymes such as UDP-N-acetylglucosamine acyltransferase and archaeal carbonic anhydrase.
[5] Other proteins that contain beta helices include the antifreeze proteins from the beetle Tenebrio molitor (right-handed)[6] and from the spruce budworm, Choristoneura fumiferana (left-handed),[7] where regularly spaced threonines on the β-helices bind to the surface of ice crystals and inhibit their growth.
Beta helices can associate with each other effectively, either face-to-face (mating the faces of their triangular prisms) or end-to-end (forming hydrogen bonds).