Developmental bioelectricity
It functions along with biochemical factors, transcriptional networks, and other physical forces to regulate cell behaviour and large-scale patterning in processes such as embryogenesis, regeneration, and cancer suppression.
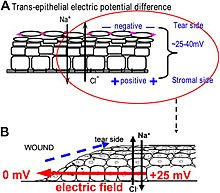
Developmental bioelectricity refers to the endogenous ion fluxes, transmembrane and transepithelial voltage gradients, and electric currents and fields produced and sustained in living cells and tissues.
[2][3] This electrical activity is often used during embryogenesis, regeneration, and cancer suppression—it is one layer of the complex field of signals that impinge upon all cells in vivo and regulate their interactions during pattern formation and maintenance.
It provided compartmentalization permitting the setting of a differential voltage/potential gradient (battery or voltage source) across the membrane, probably allowing early and rudimentary bioenergetics that fueled cell mechanisms.
Together, these voltages and electric fields form rich and dynamic and patterns inside living bodies that demarcate anatomical features, thus acting like blueprints for gene expression and morphogenesis in some instances.
More than correlations, these bioelectrical distributions are dynamic, evolving with time and with the microenvironment and even long-distant conditions to serve as instructive influences over cell behavior and large-scale patterning during embryogenesis, regeneration, and cancer suppression.
Several seminal works stimulating muscle contractions using Leyden jars culminated with the publication of classical studies by Luigi Galvani in 1791 (De viribus electricitatis in motu musculari) and 1794.
Alessandro Volta showed that the frog's leg muscle twitching was due to a static electricity generator and from dissimilar metals undergoing or catalyzing electrochemical reactions.
Applied electric fields were demonstrated to alter the regeneration of planarian by Marsh and Beams in the 1940s and 1950s,[39][40] inducing the formation of heads or tails at cut sites, reversing the primary body polarity.
In the 1970s, Lionel Jaffe and Richard Nuccittelli's introduction and development of the vibrating probe, the first device for quantitative non-invasive characterization of the extracellular minute ion currents, revitalized the field.
[41][42][43][44][45] Researchers such as Joseph Vanable, Richard Borgens, Ken Robinson, and Colin McCaig explored the roles of endogenous bioelectric signaling in limb development and regeneration, embryogenesis, organ polarity, and wound healing.
[49][50] Subsequent work has identified specific regions of the resting potential spectrum that correspond to distinct cell states such as quiescent, stem, cancer, and terminally differentiated.
[51] Although this body of work generated a significant amount of high-quality physiological data, this large-scale biophysics approach has historically come second to the study of biochemical gradients and genetic networks in biology education, funding, and overall popularity among biologists.
[53][54][11][55][23][excessive citations] The glass microelectrode was developed in the 1940s to study the action potential of excitable cells, deriving from the seminal work by Hodgkin and Huxley in the giant axon squid.
The optrode does not require referencing and is insensitive to electromagnetism[66] simplifying system setting up and making it a suitable option for recordings where electric stimulation is simultaneously applied.
Much work to functionally study bioelectric signaling has made use of applied (exogenous) electric currents and fields via DC and AC voltage-delivering apparatus integrated with agarose salt bridges.
[68] Progress in molecular biology over the last six decades has produced powerful tools that facilitate the dissection of biochemical and genetic signals; yet, they tend to not be well-suited for bioelectric studies in vivo.
Another advantage of fluorescence and other probes is their less-invasive nature and spatial multiplexing, enabling the simultaneous monitoring of large areas of embryonic or other tissues in vivo during normal or pathological pattering processes.
Screens have identified roles for ion channels in size control of structures such as the zebrafish fin,[103] while focused gain-of-function studies have shown for example that body parts can be re-specified at the organ level – for example creating entire eyes in gut endoderm.
ATS patients experience periodic paralysis, cardiac arrhythmias, and multiple morphological abnormalities that can include cleft or high arched palate, cleft or thin upper lip, flattened philtrum, micrognathia, dental oligodontia, enamel hypoplasia, delayed dentition eruption, malocclusion, broad forehead, wide set eyes, low set ears, syndactyly, clinodactyly, brachydactyly, and dysplastic kidneys.
[106][107] Mutations that disrupt another inwardly rectifying K+ channel Girk2 encoded by KCNJ6 cause Keppen-Lubinsky syndrome which includes microcephaly, a narrow nasal bridge, a high arched palate, and severe generalized lipodystrophy (failure to generate adipose tissue).
Recent research has started to identify some genetic, signaling and structural elements underlying how cells sense and respond to small physiological electric fields.
[153] Using electric fields overriding endogenous ones, Marsh and Beams astoundingly generated double-headed planarians and even reversed the primary body polarity entirely, with tails growing where a head previously existed.
[154] After these seed studies, variations of the idea that bioelectricity could sense injury and trigger or at least be a major player in regeneration have spurred over the decades until the present day.
[29][203][204] Patterning cues are often mediated by spatial gradients of cell resting potentials, or Vmem, which can be transduced into second messenger cascades and transcriptional changes by a handful of known mechanisms.
These potentials are set by the function of ion channels and pumps, and shaped by gap junctional connections which establish developmental compartments (isopotential cell fields).
The outputs of developmental bioelectric dynamics in vivo represent large-scale patterning decisions such as the number of heads in planarian,[179] the shape of the face in frog development,[99] and the size of tails in zebrafish.
[176] Recent work has shown the use of physiological modeling environments for identifying predictive interventions to target bioelectric states for repair of embryonic brain defects under a range of genetic and pharmacologically induced teratologies.
[128] Thus, the proximal mechanisms of bioelectric signaling within single cells are becoming well-understood, and advances in optogenetics[80][172][4][208][209][excessive citations] and magnetogenetics[210] continue to facilitate this research program.
The incorporation of bioelectrics with chemical signaling in the emerging field of probing cell sensory perception and decision-making[211][212][213][214][215][216][excessive citations] is an important frontier for future work.