Biomarker
[3] All four types of biomarkers have a clinical role in narrowing or guiding treatment decisions and follow a sub-categorization of being either predictive, prognostic, or diagnostic.
Predictive biomarkers are used to help optimize ideal treatments, and often indicate the likelihood of benefiting from a specific therapy.
For example, in metastatic colorectal cancer predictive biomarkers can serve as a way of evaluating and improving patient survival rates and in the individual case by case scenario, they can serve as a way of sparing patients from needless toxicity that arises from cancer treatment plans.
This marker can be measured as a proxy of prostate size with rapid changes potentially indicating cancer.
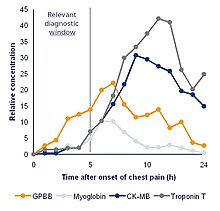
[8] An example is the traumatic brain injury (TBI) blood-based biomarker test consisted of measuring the levels of neuronal Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) and Glial fibrillary acidic protein (GFAP) to aid in the diagnosis of the presence of cranial lesion(s) among moderate to mild TBI patients that is(are) otherwise only diagnosable with the use of a CT scan of the head.
Biomarkers for precision oncology are typically utilized in the molecular diagnostics of chronic myeloid leukemia, colon, breast, and lung cancer, and in melanoma.
[11] So far, digital biomarkers have been focusing on monitoring vital parameters such as accelerometer data and heartrate[12][13] but also speech.
In Parkinson's disease (PD), for example, finger tapping a mobile phone via counting apps have been used as a method of (self-)evaluating bradykinesia and effectiveness of medication.
[17] One major current use of digital biomarkers involves keeping track of regular brain activity.
[18] While patients carryout everyday tasks (IADL), computers are using machine learning to observe and detect any deviation from normal behavior.
As illustrated by the graph, the mutation is prognostic since its presence in the patient endure the same outcome regardless of the treatment method used.
Prognostic factors are often used in combination with predictive variables in therapeutics studies, to examine how effective different treatments are in curing specific diseases or cancer.
As opposed to predictive biomarkers, prognostic do not rely on any explanatory variables, thus allowing for independent examination of the underlying disease or condition.
[22][23] Many biomarkers are derived from compounds found in foods, such as sugar or phytochemicals, or combinations thereof using a metabolomics.
Mutations in mitochondrial DNA have been shown to correlate to risk, progression, and treatment response of head and neck squamous cell carcinoma.
[28] The Early Detection Research Network (EDRN) compiled a list of seven criteria by which biomarkers can be assessed in order to streamline clinical validation.
[29] Previously used to identify the specific characteristics of the biomarker, this step is essential for doing an in situ validation of these benefits.
[30] One of the most important steps, it serves to identify specific characteristics of the candidate biomarker before developing a routine test.
In 1997 the National Institute of Health suggested a need for guidelines and legislation development that would regulate the ethical dimensions of biomarker studies.
[39] Her book raised ethical issues against chemical corporations that were controlling the general reception of the effect of pesticides on the environment, which pioneered the need for ecotoxicological studies.