Biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzyme substrates, with conversion by the living organism either into simpler or more complex products.
Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism.
Elements of biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may need coenzymes (e.g. NADH, NADPH).
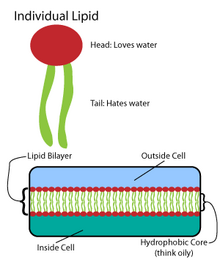
[4] These fatty acids create larger components, which in turn incorporate noncovalent interactions to form the lipid bilayer.
[4] Fatty acid chains are found in two major components of membrane lipids: phospholipids and sphingolipids.
[4] The phospholipid heads interact with each other and aqueous media, while the hydrocarbon tails orient themselves in the center, away from water.
However, the first step in phospholipid synthesis involves the formation of phosphatidate or diacylglycerol 3-phosphate at the endoplasmic reticulum and outer mitochondrial membrane.
[9] Then, lysophosphatidate is converted to phosphatidate via the addition of another fatty acid chain contributed by a second acyl CoA; all of these steps are catalyzed by the glycerol phosphate acyltransferase enzyme.
[11] Sphingolipids are formed from ceramides that consist of a fatty acid chain attached to the amino group of a sphingosine backbone.
Not only does it serve as a component of lipid membranes, it is also a precursor to several steroid hormones, including cortisol, testosterone, and estrogen.
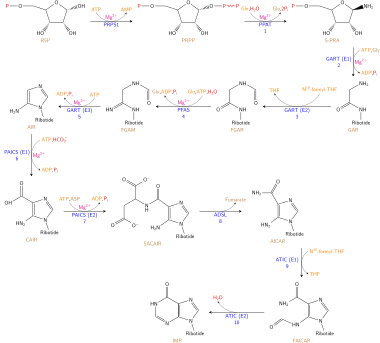
[9] The stages are as follows:[12] The biosynthesis of nucleotides involves enzyme-catalyzed reactions that convert substrates into more complex products.
[13] The DNA nucleotides adenosine and guanosine consist of a purine base attached to a ribose sugar with a glycosidic bond.
In the case of RNA nucleotides deoxyadenosine and deoxyguanosine, the purine bases are attached to a deoxyribose sugar with a glycosidic bond.
The purine bases on DNA and RNA nucleotides are synthesized in a twelve-step reaction mechanism present in most single-celled organisms.
[21] The mechanism, which depicts the reaction UTP + ATP + glutamine ⇔ CTP + ADP + glutamate, is below: Cytosine is a nucleotide that is present in both DNA and RNA.
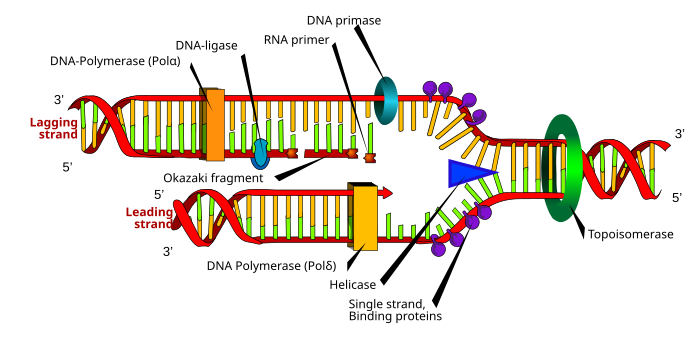
[23] During the polymerization reaction catalyzed by DNA polymerase, a nucleophilic attack occurs by the 3'OH of the growing chain on the innermost phosphorus atom of a deoxynucleoside triphosphate; this yields the formation of a phosphodiester bridge that attaches a new nucleotide and releases pyrophosphate.
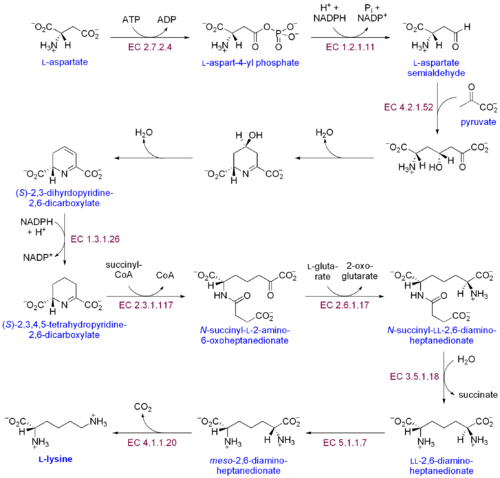
The other amino acids, valine, methionine, leucine, isoleucine, phenylalanine, lysine, threonine and tryptophan for adults and histidine, and arginine for babies are obtained through diet.
[24] One major step in amino acid biosynthesis involves incorporating a nitrogen group onto the α-carbon.
The other pathway for incorporating nitrogen onto the α-carbon of amino acids involves the enzyme glutamate dehydrogenase (GDH).
Subsequent steps are catalyzed by the enzymes N-acetylglutamate kinase, N-acetyl-gamma-glutamyl-phosphate reductase, and acetylornithine/succinyldiamino pimelate aminotransferase and yield the N-acetyl-L-ornithine.
[27] During serine biosynthesis,[34] the enzyme phosphoglycerate dehydrogenase catalyzes the initial reaction that oxidizes 3-phospho-D-glycerate to yield 3-phosphonooxypyruvate.
[35] The following reaction is catalyzed by the enzyme phosphoserine aminotransferase, which transfers an amino group from glutamate onto 3-phosphonooxypyruvate to yield L-phosphoserine.
[36] The final step is catalyzed by the enzyme phosphoserine phosphatase, which dephosphorylates L-phosphoserine to yield L-serine.
Organisms that use ethanol and acetate as the major carbon source utilize the glyconeogenic pathway to synthesize glycine.
In microorganisms and plants, the enzyme serine acetyltransferase catalyzes the transfer of acetyl group from acetyl-CoA onto L-serine to yield O-acetyl-L-serine.
[39] The following reaction step, catalyzed by the enzyme O-acetyl serine (thiol) lyase, replaces the acetyl group of O-acetyl-L-serine with sulfide to yield cysteine.
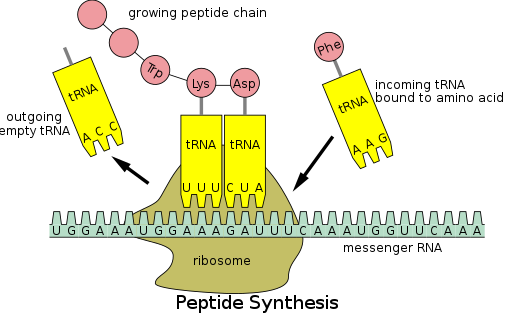
[53] During translation, genetic material called mRNA is read by ribosomes to generate a protein polypeptide chain.
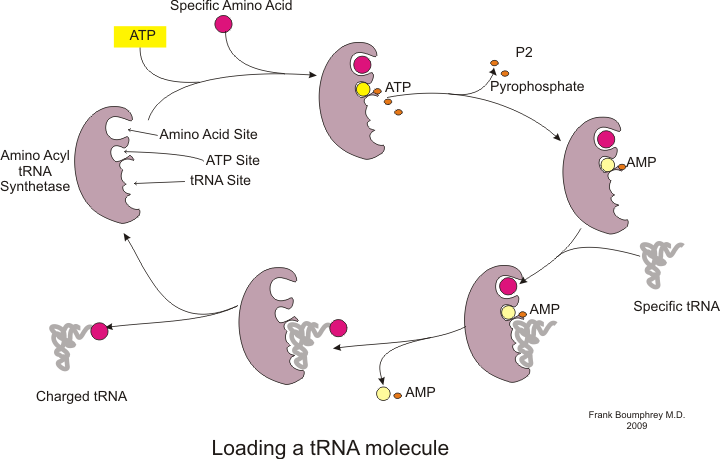
[53] This process requires transfer RNA (tRNA) which serves as an adaptor by binding amino acids on one end and interacting with mRNA at the other end; the latter pairing between the tRNA and mRNA ensures that the correct amino acid is added to the chain.
[54] Furthermore, this enzyme has special discriminator regions to ensure the correct binding between tRNA and its cognate amino acid.
The recognition of codons by release factors, which causes the hydrolysis of the polypeptide chain from the tRNA located in the P site[1] 2.