Bismuth(III) sulfide
Bismuth(III) sulfide can be prepared by reacting a bismuth(III) salt with hydrogen sulfide: Bismuth (III) sulfide can also be prepared by the reaction of elemental bismuth and elemental sulfur in an evacuated silica tube at 500 °C for 96 hours.
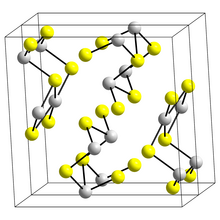
Bismuth(III) sulfide is isostructural with stibnite (stibnite is one of the forms of antimony(III) sulfide).
Bismuth atoms are in two different environments, both of which have 7 coordinate Bismuth atoms, 4 in a near planar rectangle and three more distant making an irregular 7-coordination group.
[2] It can react with acids to produce the odoriferous hydrogen sulfide gas.
It is used as a starting material to produce many other bismuth compounds.