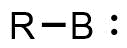Borylene
[10] As discussed above, free borylenes have yet to be isolated, but they have been the subject of a number of computational studies and have investigated spectroscopically and experimentally.
B-R (R=H, F, Cl, Br, I, NH2, C2H, Ph) have been observed via microwave or IR spectroscopy at low temperature via elaborate procedures.
[13][14][15][16] When generated as reactive intermediates, borylenes have been shown to activate strong C-C single bonds, yielding products analogous to an organometallic oxidative addition reaction.
As might be expected, calculations have demonstrated that the HOMO is composed of the nonbonding electrons on boron (nσ-type, sp character).
The LUMO and LUMO+1 are empty, orthogonal pπ-type orbitals and are degenerate in energy except in the case where R breaks the symmetry of the molecule, thus lifting the degeneracy.
Aminoborylene (H2NB) is a slight exception to the above paradigm, as the nitrogen lone pair donates into an unoccupied boron p orbital.
Although some differences between the calculated and crystal structures were evident, they could primarily be ascribed to distortions from planarity caused by the bulky Dipp groups.
[18] A number of similar compounds have been generated and isolated, and several studies involving putative mono-Lewis base-stabilized borylene intermediates have been reported.
[19] Betrand et al. argued that due to boron's electropositivity and thus preference to be electron-poor, CAAC (cyclic (alkyl)(amino)carbene) might serve as a better Lewis base than the more commonplace NHC.
As previously discussed, a nitrogen lone pair donates into an empty boron p-orbital to form a π bond; the out of phase combination serves as a high-energy LUMO+2.
Taking inspiration from Robinson's above diborene synthesis,[21][18] Bertrand et al. swapped NHC for CAAC and successfully isolated the first bis-Lewis base-stabilized borylene in 2011.
Reduction of (CAAC)BBr3 yields the same terminal borylene even in the absence of additional Lewis base via a mechanism that remains poorly understood.
At least one π-acceptor ligand is present in all known examples of these compounds, and the B-L bond strength tends to scale with the π-acidity of the Lewis base.
[28] The first transition metal complex reported by Braunschweig et al. featured a borylene ligand bridging between two manganese centers: [ μ-BX{η5-C5H4R}Mn(CO)2}2] (R=H, Me; X=NMe2).