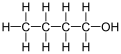
1-Butanol
1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides[6] and is present in many foods and drinks.
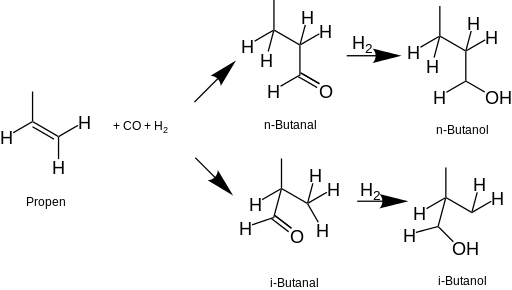
[8] Since the 1950s, most 1-butanol is produced by the hydroformylation of propene (oxo process) to preferentially form the butyraldehyde n-butanal.
Research in the past few decades showed results of other microorganisms that can produce butanol through fermentation.
[12] The production of, or in some cases, the use of, the following substances may result in exposure to 1-butanol: artificial leather, butyl esters, rubber cement, dyes, fruit essences, lacquers, motion picture, and photographic films, raincoats, perfumes, pyroxylin plastics, rayon, safety glass, shellac varnish, and waterproofed cloth.
[7] Butan-1-ol occurs naturally as a result of carbohydrate fermentation in a number of alcoholic beverages, including beer,[13] grape brandies,[14] wine,[15] and whisky.
[16] It has been detected in the volatiles of hops,[17] jack fruit,[18] heat-treated milks,[19] musk melon,[20] cheese,[21] southern pea seed,[22] and cooked rice.
[26][27] It (along with similar fusel alcohols) is reputed to be responsible for severe hangovers, although experiments in animal models show no evidence for this.
[28] 1-Butanol is used as an ingredient in processed and artificial flavorings,[29] and for the extraction of lipid-free protein from egg yolk,[30] natural flavouring materials and vegetable oils, the manufacture of hop extract for beermaking, and as a solvent in removing pigments from moist curd leaf protein concentrate.
Butyric acid can be fully metabolized to carbon dioxide and water by the β-oxidation pathway.
[33] At sub-lethal doses, 1-butanol acts as a depressant of the central nervous system, similar to ethanol: one study in rats indicated that the intoxicating potency of 1-butanol is about 6 times higher than that of ethanol, possibly because of its slower transformation by alcohol dehydrogenase.