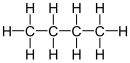
Butane
Both isomers are highly flammable, colorless, easily liquefied gases that quickly vaporize at room temperature and pressure.
Normal butane can be used for gasoline blending, as a fuel gas, fragrance extraction solvent, either alone or in a mixture with propane, and as a feedstock for the manufacture of ethylene and butadiene, a key ingredient of synthetic rubber.
[21][22][23][24] For gasoline blending, n-butane is the main component used to manipulate the Reid vapor pressure (RVP).
It is used as a petrol component, as a feedstock for the production of base petrochemicals in steam cracking, as fuel for cigarette lighters and as a propellant in aerosol sprays such as deodorants.
In the 20th century, the Braun company of Germany made a cordless hair styling device product that used butane as its heat source to produce steam.
[29] As fuel, butane is often mixed with small amounts of mercaptans to give the unburned gas an offensive smell easily detected by the human nose.
If not removed, it will otherwise leave a deposit at the point of ignition and may eventually block the uniform flow of gas.
Inhalation of butane can cause euphoria, drowsiness, unconsciousness, asphyxia, cardiac arrhythmia, fluctuations in blood pressure and temporary memory loss, when abused directly from a highly pressurized container, and can result in death from asphyxiation and ventricular fibrillation.
[35] Butane is the most commonly abused volatile substance in the UK, and was the cause of 52% of solvent related deaths in 2000.
[36] By spraying butane directly into the throat, the jet of fluid can cool rapidly to −20 °C (−4 °F) by expansion, causing prolonged laryngospasm.