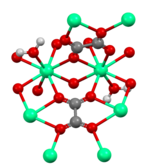
Calcium oxalate
[9] The poisonous plant dumb cane (Dieffenbachia) contains the substance and on ingestion can prevent speech and be suffocating.
Insoluble calcium oxalate crystals are found in plant stems, roots, and leaves and produced in idioblasts.
Vanilla plants exude calcium oxalates upon harvest of the orchid seed pods and may cause contact dermatitis.
Calcium oxalate crystals are commonly found in lichens, where they occur in two mineral forms: weddellite (CaC2O4·(2+x)H2O) and whewellite (CaC2O4·H2O).
The type and distribution of these crystals often correlates with environmental conditions: weddellite typically forms in dry environments and can serve as a water source for the lichen, while whewellite is more common in moist habitats.
In addition to water regulation, calcium oxalate crystals in lichens serve several protective functions, including shielding against excessive sunlight and potentially helping to neutralize pollutants such as sulfur dioxide.
[10] Calcium oxalate, as ‘beerstone’, is a brownish precipitate that tends to accumulate within vats, barrels, and other containers used in the brewing of beer.
[11] Beerstone is composed of calcium and magnesium salts and various organic compounds left over from the brewing process; it promotes the growth of unwanted microorganisms that can adversely affect or even ruin the flavour of a batch of beer.