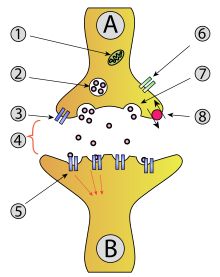
Calyx of Held
[5][6][7] These principal cells then project to the ipsilateral lateral superior olive (LSO),[8] where they inhibit postsynaptic neurons and provide a basis for interaural level detection (ILD), required for high frequency sound localization.
[10] The related endbulb of Held is also a large axon terminal synapse (15–30 μm in diameter) found in another auditory brainstem structure, namely the anteroventral cochlear nucleus (AVCN).
[12] Commonly used in research due to its large size, the calyx of Held has been used to understand a variety of mechanisms related to development of, and vesicle release of the synapse.
As a synapse, the function of the calyx of Held is to transmit the signal from the GBCs to the principal neurons of the MNTB, which are glycinergic, thus hyperpolarizing cells in the lateral superior olive (LSO) nuclei and producing inhibitory effects.
[12] As a result of its role in stimulating the principal neurons of the MNTB, the primary function of the calyx of Held is to allow differentiation between temporal activation of the cochlear hair cells that are important in sound localization (interaural level detection).
The large diameter of the bushy cell axons allows the inhibitory signal produced by the MNTB neurons to reach the SOC approximately 0.2 ms later than the ipsilateral excitation.
These microtubules carry out a variety of functions, such as providing stability to the synapse, restricting the distribution of the synaptic vesicles, and localizing the mitochondria.
[12] At postnatal day two (P2), the immature rat calyx of Held is formed, easily distinguished by its characteristic sealed-spoon morphology.
[12] The primary synaptic contacts that form the calyx are assembled between neurons of the MNTB (medial nucleus of the trapezoid body) and VCN (ventral cochlear nerve), eventually connecting with one another by projecting across the midline of the two areas.
These associations begin to appear immediately after VCN neurons have been generated; one can observe the earliest formation of these contacts around embryonic day 17 (E17).
The combined effects of these three molecules with one another illustrate the fact that there are many families of proteins involved in proper signaling and formation of the calyx.
[14] Additionally, Eph proteins are integral for further auditory circuit system development after initial embryonic calyx formation.
The second ultrastructural change involves the principal neurons of the MNTBs, whose cell bodies and nuclei increase in surface area due to enlargement.
Evidence shows that the expression in the alpha subunit of NaV1.6, a specific type of sodium channel, is responsible for the increased speed in transmission.
[14] In order to compensate for the myelination (increased capacitance), leading up to the calyx at the last node (the area between the myelin sheath) before the axon terminal contains a high density of Na+ channels in order to allow a large influx (inward flow) of sodium to trigger the voltage-gated calcium channels to open in the presynaptic terminal, causing a calcium influx.
[12] Between the second and third postnatal weeks, around the time of hearing onset, the calyx of Held develops its characteristic, highly fenestrated (many openings) appearance.
[14] Fenestration results in the membrane being reduced to numerous small compartments, which increases surface-to-volume ratio of the calyx of Held.
Upon calcium influx, a cAMP-response element binding protein (CREB) is phosphorylated, altering the potassium concentrations within the cell to return the terminal to an inactive state.
As an excitatory neurotransmitter, glutamate almost always causes an action potential to be triggered on the postsynaptic side – further encouraged by low internal sodium of the principal neurons.
[12] In the mature calyx, the AMPA receptors are concentrated on the principal neuron as to localize the transmission for greater action potential probability.