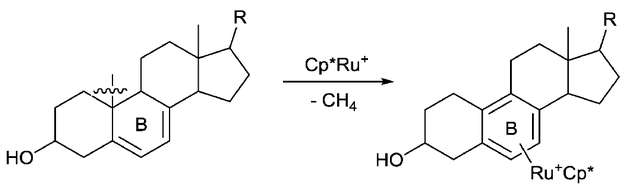
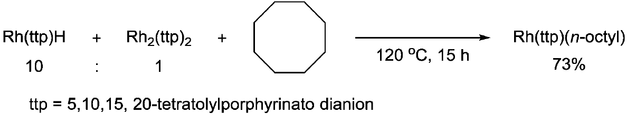
Carbon-carbon bond activation
One example is reported by Milstein and coworkers, in which the C(sp2)–C(sp3) bond of bisphosphine ligands was selectively cleaved by a number of metals to afford stable pincer complexes under mild conditions.
Chan group reported the C–C bond scission of cyclooctane via 1,2-addition with Rh(III) porphyrin hydride, which involved [RhII(ttp)]· radical as the key intermediate.
[8] Generally speaking, there are two distinct mechanistic pathways that lead to C-C bond activation: (a) the β-carbon elimination of metal complexes.
The early stage of research in this field has focused on the reaction of M–O–C–C species and β-carbon elimination of the M–N–C–C intermediate was not discovered until the recent ten years.
For example, in 2021 Dong Group described the first enantioselective total synthesis of the natural product penicibilaenes using a late-stage carbon-carbon bond activation strategy.
[12] There are also a lot of other examples highlighting the potential of carbon-carbon bond activation strategies in the total synthesis of complex natural products with high stereocontrol.