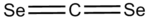Carbon diselenide
Carbon diselenide is an inorganic compound with the chemical formula CSe2.
This light-sensitive compound is insoluble in water and soluble in organic solvents.
It is produced by reacting selenium powder with dichloromethane vapor near 550 °C.
[1] It was first reported by Grimm and Metzger, who prepared it by treating hydrogen selenide with carbon tetrachloride in a hot tube.
[5] Pure distilled carbon diselenide has an odor very similar to that of carbon disulfide, but mixed with air, it creates extremely offensive odors (corresponding to new, highly toxic reaction products).