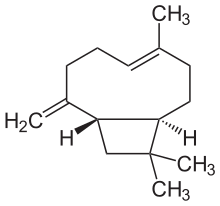
Caryophyllene
[3] Caryophyllene can be produced synthetically,[4] but it is invariably obtained from natural sources because it is widespread.
[6] In other basic studies, β-caryophyllene has a binding affinity of Ki = 155 nM at the CB2 receptors.
[16] The metabolism of caryophyllene progresses through (−)-caryophyllene oxide (C15H24O) since the latter compound also afforded 14-hydroxycaryophyllene (C15H24O) as a metabolite.
This further reacts with a second unit of IPP, also via an SN1-type reaction catalyzed by the enzyme IspA, to form farnesyl pyrophosphate (FPP).
Finally, FPP undergoes QHS1 enzyme-catalyzed intramolecular cyclization to form caryophyllene.