Phosphatidylethanolamine
[2] Phosphatidylethanolamines play a role in membrane fusion and in disassembly of the contractile ring during cytokinesis in cell division.
[2] As a polar head group, phosphatidylethanolamine creates a more viscous lipid membrane compared to phosphatidylcholine.
[5] Phosphatidylethanolamine has also shown to be able to propagate infectious prions without the assistance of any proteins or nucleic acids, which is a unique characteristic of it.
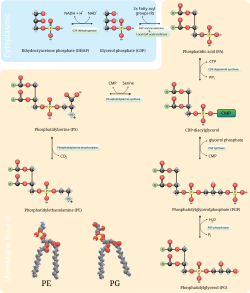
The phosphatidylserine decarboxylation pathway is the main source of synthesis for phosphatidylethanolamine in the membranes of the mitochondria.
In a process that mirrors phosphatidylcholine synthesis, phosphatidylethanolamine is also made via the cytidine diphosphate-ethanolamine pathway, using ethanolamine as the substrate.
Through several steps taking place in both the cytosol and endoplasmic reticulum, the synthesis pathway yields the end product of phosphatidylethanolamine.
[11] Phosphatidylethanolamine is also found abundantly in soy or egg lecithin and is produced commercially using chromatographic separation.
[12] Phosphatidylethanolamines in food break down to form phosphatidylethanolamine-linked Amadori products as a part of the Maillard reaction.
[13] These products accelerate membrane lipid peroxidation, causing oxidative stress to cells that come in contact with them.