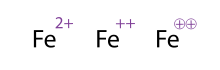Ion
Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds.
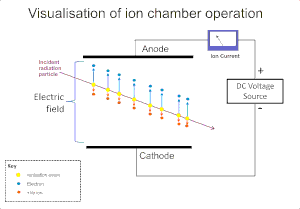
[5] Ions are also created by chemical interactions, such as the dissolution of a salt in liquids, or by other means, such as passing a direct current through a conducting solution, dissolving an anode via ionization.
The word ion was coined from neuter present participle of Greek ἰέναι (ienai), meaning "to go".
In correspondence with Faraday, Whewell also coined the words anode and cathode, as well as anion and cation as ions that are attracted to the respective electrodes.
[6] Svante Arrhenius put forth, in his 1884 dissertation, the explanation of the fact that solid crystalline salts dissociate into paired charged particles when dissolved, for which he would win the 1903 Nobel Prize in Chemistry.
Thus, anions (negatively charged ions) are larger than the parent molecule or atom, as the excess electron(s) repel each other and add to the physical size of the ion, because its size is determined by its electron cloud.
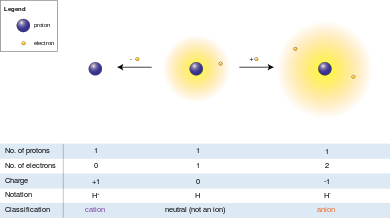
[14] A cation (+) (/ˈkætˌaɪ.ən/ KAT-eye-ən, from the Greek word κάτω (kátō), meaning "down"[15]) is an ion with fewer electrons than protons, giving it a positive charge.
"[18] The terms anion and cation (for ions that respectively travel to the anode and cathode during electrolysis) were introduced by Michael Faraday in 1834 following his consultation with William Whewell.
Ions are ubiquitous in nature and are responsible for diverse phenomena from the luminescence of the Sun to the existence of the Earth's ionosphere.
In both inorganic and organic chemistry (including biochemistry), the interaction of water and ions is often relevant for understanding properties of systems; an example of their importance is in the breakdown of adenosine triphosphate (ATP), which provides the energy for many reactions in biological systems.
As reactive charged particles, they are also used in air purification by disrupting microbes, and in household items such as smoke detectors.
This is a common mechanism exploited by natural and artificial biocides, including the ion channels gramicidin and amphotericin (a fungicide).
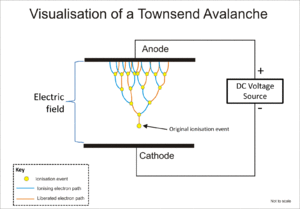
[5] The Geiger–Müller tube and the proportional counter both use a phenomenon known as a Townsend avalanche to multiply the effect of the original ionizing event by means of a cascade effect whereby the free electrons are given sufficient energy by the electric field to release further electrons by ion impact.
However, it is possible to mix the notations for the individual metal centre with a polyatomic complex, as shown by the uranyl ion example.
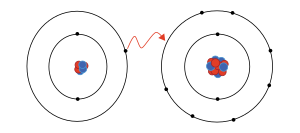
The process of gaining or losing electrons from a neutral atom or molecule is called ionization.
This transfer is usually driven by the attaining of stable ("closed shell") electronic configurations.
For example, when ammonia, NH3, accepts a proton, H+—a process called protonation—it forms the ammonium ion, NH+4.
Ammonia can also lose an electron to gain a positive charge, forming the ion NH+3.
This allows the molecule to preserve its stable electronic configuration while acquiring an electrical charge.
For this reason, ions tend to form in ways that leave them with full orbital blocks.
[19] In general, the ionization energy of metals is much lower than the ionization energy of nonmetals, which is why, in general, metals will lose electrons to form positively charged ions and nonmetals will gain electrons to form negatively charged ions.
This reaction produces metal cations and nonmetal anions, which are attracted to each other to form a salt.