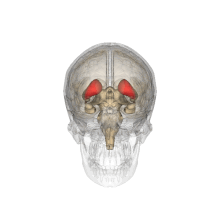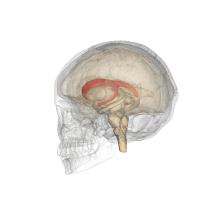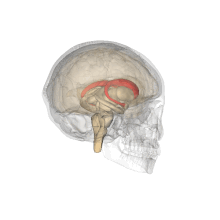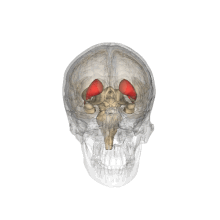
Caudate nucleus
Each nucleus is C-shaped, with a wider "head" (caput in Latin) at the front, tapering to a "body" (corpus) and a "tail" (cauda).
This means that a coronal section (on a plane parallel to the face) that cuts through the tail will also cross the body and head of the caudate nucleus.
With this in mind, the caudate nucleus could be involved in the recruitment of the motor system to support working memory performance by the mediation of sensory-motor transformations.
A delay in initiating performance and the need to shift body position constantly were both observed in cats after partial removal of the nuclei.
The "motor release" caused by this procedure indicates that the caudate nucleus inhibits the tendency for an animal to move forward without resistance.
A two-pronged approach of neuroimaging (including PET and fMRI) and anatomical studies expose a strong relationship between the caudate and cortical areas associated with executive functioning: "non-invasive measures of anatomical and functional connectivity in humans demonstrate a clear link between the caudate and executive frontal areas.
[15] While here the choice was far more complex––the subjects were not simply asked to press a lever, but had to weigh a host of different factors––at the crux of the study was still behavioral selection based on changing values of outcomes.
The dorsal-prefrontal cortex subcortical loop involving the caudate nucleus has been linked to deficits in working memory, specifically in schizophrenic patients.
Functional imaging has shown activation of this subcortical loop during working memory tasks in primates and healthy human subjects.
Caudate nucleus volume has been found to be inversely associated with perseverative errors on spatial working memory tasks.
[19] Bilateral lesions in the head of the caudate nucleus in cats were correlated with a decrease in the duration of deep slow wave sleep during the sleep-wakefulness cycle.
This discovery may be due to the inter-related nature of the roles of the caudate nucleus and the frontal cortex in controlling levels of central nervous system activation.
The cats with caudate removal, although permanently hyperactive, had a significant decrease in rapid eye movement sleep (REMS) time, which lasted about two months.
Cats with bilateral removal of the caudate nuclei persistently approached and followed objects, attempting to contact the target, while exhibiting a friendly disposition by the elicitation of treading of the forelimbs and purring.
In short, "these and other findings with bilingual patients suggest that the left caudate is required to monitor and control lexical and language alternatives in production tasks.
There is some indirect evidence[29] that the caudate may perform this regulatory role by measuring the general activity of cerebral cortex and controlling the threshold potential.
[30][41] Less common risk factors may include non-valvular atrial fibrillation, myocardial dyskinesia, cardiac aneurysm with a mural thrombus, syphilis, Hodgkin's lymphoma, and Moyamoya disease.
[30][41] Additionally, a case report of lacunar infarction of the caudate and adjacent structures due to high-dose oral methylphenidate use has been published.
[12][31][30] In studies of patients with caudate infarcts, frequently occurring symptoms have included dysarthria or dysphonia (61–86%), motor weakness (40–100%), and cognitive/behavioral abnormalities (39–78%), including abulia (26–48%), agitation (29%), restlessness, hyperactivity, disinhibition (9–11%), executive dysfunction or frontal system abnormalities (26%), memory impairment, minor speech or linguistic deficits (23–50% of left-sided lesions), attention difficulties, and mood changes or depression (14–33%).
[12][31][30][54][55] In one case report of bilateral caudate head damage, severe prospective memory impairment was measured, along with other deficits.
[41][30] However, patients with caudate strokes can have residual deficits and dependency needs, can worsen clinically with time, or can require institutionalization.
[30] On the basis of case reports and small case series, disorders of diminished motivation, like apathy, abulia, and akinetic mutism, secondary to stroke and other causes, may be treated with dopaminergic agents and other pro-motivational medications, including psychostimulants, bupropion, atomoxetine, modafinil, dopamine agonists, levodopa, selegiline, and acetylcholinesterase inhibitors.
Patients with this progressive neurodegenerative disorder often first experience movement related symptoms (the three most common being tremors at rest, muscular rigidity, and akathisia) which are later combined with various cognitive deficiencies, including dementia.
[69] Parkinson's disease depletes dopaminergic neurons in the nigrostriatal tract, a dopamine pathway that is connected to the head of the caudate.
As such, many studies have correlated the loss of dopaminergic neurons that send axons to the caudate nucleus and the degree of dementia in Parkinson's patients.
A 2004 study uses magnetic resonance imaging to compare the relative volume of white matter in the caudate among schizophrenia patients.
Those patients with the disorder have "smaller absolute and relative volumes of white matter in the caudate nucleus than healthy subjects.
[75][76] It has been theorized that the caudate nucleus may be dysfunctional in persons with obsessive compulsive disorder (OCD), in that it may perhaps be unable to properly regulate the transmission of information regarding worrying events or ideas between the thalamus and the orbitofrontal cortex.
A neuroimaging study with positron emission tomography found that the right caudate nucleus had the largest change in glucose metabolism after patients had been treated with paroxetine.
[77] Recent SDM meta-analyses of voxel-based morphometry studies comparing people with OCD and healthy controls have found people with OCD to have increased grey matter volumes in bilateral lenticular nuclei, extending to the caudate nuclei, while decreased grey matter volumes in bilateral dorsal medial frontal/anterior cingulate gyri.