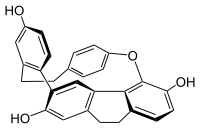
Cavicularin
Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa.
This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity solely due to the presence of planar chirality and axial chirality.
The para-substituted phenol ring is bent about 15° out of planarity, adopting a somewhat boat-like geometry.
This type of angle strain in aromatic compounds is normally reserved for synthetic cyclophanes.
The material was dried for one day, ground to a powder and 5 grams were refluxed in methanol for 4 months [reference needed] to yield 2.5 mg (0.049%) of cavicularin after column chromatography and preparative TLC.