Cellulose
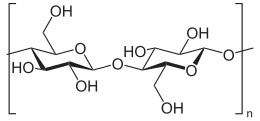
Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units.
[3][4] Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes.
[10] Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha.
In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.
Cellulose was discovered in 1838 by the French chemist Anselme Payen, who isolated it from plant matter and determined its chemical formula.
[13] Cellulose has no taste, is odorless, is hydrophilic with the contact angle of 20–30 degrees,[14] is insoluble in water and most organic solvents, is chiral and is biodegradable.
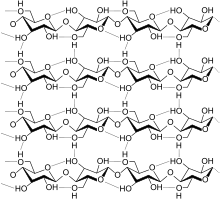
This confers tensile strength in cell walls where cellulose microfibrils are meshed into a polysaccharide matrix.
The high tensile strength of plant stems and of the tree wood also arises from the arrangement of cellulose fibers intimately distributed into the lignin matrix.
[17] Live fluorescence microscopy techniques are promising in investigation of the role of cellulose in growing plant cells.
[20] Many properties of cellulose depend on its chain length or degree of polymerization, the number of glucose units that make up one polymer molecule.
[24] These nanocelluloses are of high technological interest due to their self-assembly into cholesteric liquid crystals,[25] production of hydrogels or aerogels,[26] use in nanocomposites with superior thermal and mechanical properties,[27] and use as Pickering stabilizers for emulsions.
There are known to be about seven subfamilies in the plant CesA superfamily, some of which include the more cryptic, tentatively-named Csl (cellulose synthase-like) enzymes.
[35] Cellulolysis is the process of breaking down cellulose into smaller polysaccharides called cellodextrins or completely into glucose units; this is a hydrolysis reaction.
Because cellulose molecules bind strongly to each other, cellulolysis is relatively difficult compared to the breakdown of other polysaccharides.
[41] At temperatures above 350 °C, cellulose undergoes thermolysis (also called 'pyrolysis'), decomposing into solid char, vapors, aerosols, and gases such as carbon dioxide.
[44] Glycosidic bond cleavage produces short cellulose chains of two-to-seven monomers comprising the melt.
Vapor bubbling of intermediate liquid cellulose produces aerosols, which consist of short chain anhydro-oligomers derived from the melt.
[45] Continuing decomposition of molten cellulose produces volatile compounds including levoglucosan, furans, pyrans, light oxygenates, and gases via primary reactions.
In contrast to cellulose, hemicelluloses are derived from several sugars in addition to glucose, especially xylose but also including mannose, galactose, rhamnose, and arabinose.
[51] This process, patented by the founders of the Viscose Development Company, is the most widely used method for manufacturing regenerated cellulose products.
While the first application of regenerated cellulose was as a clothing textile, this class of materials is also used in the production of disposable medical devices as well as fabrication of artificial membranes.
[64][65][66] Thiolated cellulose derivatives (see thiomers) exhibit also high binding properties for metal ions.
[74] Typical non-food energy crops include industrial hemp, switchgrass, Miscanthus, Salix (willow), and Populus (poplar) species.
A strain of Clostridium bacteria found in zebra dung, can convert nearly any form of cellulose into butanol fuel.