Central nervous system effects from radiation exposure during spaceflight
HZE nuclei are capable of producing a column of heavily damaged cells, or a microlesion, along their path through tissues, thereby raising concern over serious impacts on the CNS.
First, exposure to HZE nuclei at low doses (<50 cGy) significantly induces neurocognitive deficits, such as learning and behavioral changes as well as operant reactions in the mouse and rat.
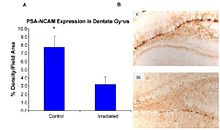
Second, exposure to HZE disrupts neurogenesis in mice at low doses (<1 Gy), showing a significant dose-related reduction of new neurons and oligodendrocytes in the subgranular zone (SGZ) of the hippocampal dentate gyrus.
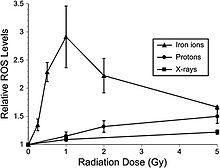
Third, reactive oxygen species (ROS) in neuronal precursor cells arise following exposure to HZE nuclei and protons at low dose, and can persist for several months.
Research with animal models that are irradiated with HZE nuclei has shown that important changes to the CNS occur with the dose levels that are of concern to NASA.
[8][9] The high energies of GCRs allow them to penetrate to hundreds of centimeters of any material, thus precluding radiation shielding as a plausible mitigation measure to GCR risks on the CNS.
[14][15] These numbers do not include the additional cell hits by energetic electrons (delta rays) that are produced along the track of HZE nuclei [13] or correlated cellular damage.
This is largely due to the lack of a human epidemiological basis with which to estimate risks and the relatively small number of published experimental studies with animals.
The effect of the protracted exposure of the CNS to the low dose-rate (< 50 mGy h–1) of proton, HZE particles, and neutrons of the relevant energies for doses up to 2 Gy is of concern.
The CNS PELs, which correspond to the doses at the region of the brain called the hippocampus, are set for time periods of 30 days or 1 year, or for a career with values of 500, 1,000, and 1,500 mGy-Eq, respectively.
[33][34] Since cognitive functioning and memory are closely associated with the cerebral white volume of the prefrontal/frontal lobe and cingulate gyrus, defects in neurogenesis may play a critical role in neurocognitive problems in irradiated patients.
[3] The first proposal concerning the effect of space radiation on the CNS was made by Cornelius Tobias in his 1952 description of light flash phenomenon caused by single HZE nuclei traversals of the retina.
The microlesion concept, which considered the effects of the column of damaged cells surrounding the path of an HZE nucleus traversing critical regions of the brain, originated at this time.
Second, when astronauts are traveling in LEO, they are partially protected by the magnetic field and the solid body of the Earth, which together reduce the GCR dose-rate by about two-thirds from its free space values.
[19] In recent years, studies with stem cells uncovered that neurogenesis still occurs in the adult hippocampus, where cognitive actions such as memory and learning are determined.
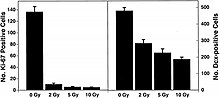
These studies demonstrate that HZE radiation led to the progressive loss of neuronal progenitor cells in the SGZ at doses of 1 to 3 Gy in a dose-dependent manner.
153 [3] notes that: “Relative ROS levels were increased at nearly all doses (1 to 10 Gy) of Bragg-peak 250 MeV protons at post-irradiation times (6 to 24 hours) compared to unirradiated controls.
Neuroinflammation, which is a fundamental reaction to brain injury, is characterized by the activation of resident microglia and astrocytes and local expression of a wide range of inflammatory mediators.
COX-2 up-regulation in irradiated microglia cells leads to prostaglandin E2 production, which appears to be responsible for radiation-induced gliosis (overproliferation of astrocytes in damaged areas of the CNS).
[58] A negative result was reported by Pecaut et al.,[59] in which no behavioral effects were seen in female C57/BL6 mice in a 2- to 8-week period following their exposure to 0, 0.1, 0.5 or 2 Gy accelerated 56Fe-ions (1 GeV/u56Fe) as measured by open-field, rotorod, or acoustic startle habituation.
[60][61][62][63][64] The CTA test is a classical conditioning paradigm that assesses the avoidance behavior that occurs when the ingestion of a normally acceptable food item is associated with illness.
[61][62] Doses as low as ~0.2 Gy of 56Fe-ions appear to have an effect on CTA.” The RBE of different types of heavy particles on CNS function and cognitive/behavioral performance was studied in Sprague-Dawley rats.
Recent studies by Rabin et al.[67] have examined the ability of rats to perform an operant order to obtain food reinforcement using an ascending fixed ratio (FR) schedule.
[69] To determine whether these findings related to brain-region specific alterations in sensitivity to oxidative stress, inflammation or neuronal plasticity, three regions of the brain, the striatum, hippocampus and frontal cortex that are linked to behavior, were isolated and compared to controls.
[63] The acute effects on the CNS, which are associated with increases in cytokines and chemokines, may lead to disruption in the proliferation of stem cells or memory loss that may contribute to other degenerative diseases.
New technologies, such as three-dimensional cell cultures, microarrays, proteomics, and brain imaging, are used in systematic studies on CNS risks from different radiation types.
Cdk5 is a kinase that plays a key role in neural development; its aberrant expression and activation are associated with neurodegenerative processes, including Alzheimer's disease.
[76] Some recent experiments suggest that, at least for acute high-dose irradiation, an efficient radioprotection by dietary supplements can be achieved, even in the case of exposure to high-LET radiation.
Existing animal and cellular data do suggest that space radiation can produce neurological and behavioral effects; therefore, it is possible that mission operations will be impacted.
A vigorous research program, which will be required to solve these problems, must rely on new approaches to risk assessment and countermeasure validation because of the absence of useful human radio-epidemiology data in this area.