Commission on Isotopic Abundances and Atomic Weights
[2] The biennial CIAAW Standard Atomic Weights are accepted as the authoritative source in science and appear worldwide on the periodic table wall charts.
In addition, until 2019 the definition of the Kelvin, the SI unit for thermodynamic temperature, made direct reference to the isotopic composition of oxygen and hydrogen as recommended by CIAAW.
[5] Although the atomic weight had taken on the concept of a constant of nature like the speed of light, the lack of agreement on accepted values created difficulties in trade.
[7] In 1897, the German Society of Chemistry, following a proposal by Hermann Emil Fischer, appointed a three-person working committee to report on atomic weights.
On 30 March 1899 Landolt, Ostwald and Seubert issued an invitation to other national scientific organizations to appoint delegates to the International Committee on Atomic Weights.
[9] In 1902, Prof. Frank W. Clarke (USA), Prof. Karl Seubert (Germany), and Prof. Thomas Edward Thorpe (UK) were elected, and the International Committee on Atomic Weights published its inaugural report in 1903 under the chairmanship of Prof.
[10] Since 1899, the Commission periodically and critically evaluates the published scientific literature and produces the Table of Standard Atomic Weights.
[15] In the early 20th century, measurements of the atomic weight of lead showed significant variations depending on the origin of the sample.
At the Commission’s meeting in 1951, it was recognized that the isotopic-abundance variation of sulfur had a significant effect on the internationally accepted value of an atomic weight.
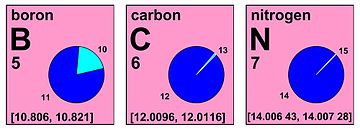
At its meeting in 2009 in Vienna, the Commission decided to express the standard atomic weight of hydrogen, carbon, oxygen, and other elements in a manner that clearly indicates that the values are not constants of nature.
Richards was awarded the 1914 Nobel Prize in Chemistry "in recognition of his accurate determinations of the atomic weight of a large number of chemical elements"[23] while he was a member of the Commission.