Actinium
[9] After a comparison of the substances' half-lives determined by Debierne,[10] Harriet Brooks in 1904, and Otto Hahn and Otto Sackur in 1905, Debierne's chosen name for the new element was retained because it had seniority, despite the contradicting chemical properties he claimed for the element at different times.
Furthermore, the now-known chemistry of actinium precludes its presence as anything other than a minor constituent of Debierne's 1899 and 1900 results; in fact, the chemical properties he reported make it likely that he had, instead, accidentally identified protactinium, which would not be discovered for another fourteen years, only to have it disappear due to its hydrolysis and adsorption onto his laboratory equipment.
[5] Debierne, who is now considered by the vast majority of historians as the discoverer, lost interest in the element and left the topic.
Giesel, on the other hand, can rightfully be credited with the first preparation of radiochemically pure actinium and with the identification of its atomic number 89.
[13] The name actinium originates from the Ancient Greek aktis, aktinos (ακτίς, ακτίνος), meaning beam or ray.
[19] Owing to its strong radioactivity, actinium glows in the dark with a pale blue light, which originates from the surrounding air ionized by the emitted energetic particles.
[20] Actinium has similar chemical properties to lanthanum and other lanthanides, and therefore these elements are difficult to separate when extracting from uranium ores.
The actinides are much more diverse than the lanthanides[22] and therefore it was not until 1945 that the most significant change to Dmitri Mendeleev's periodic table since the recognition of the lanthanides, the introduction of the actinides, was generally accepted after Glenn T. Seaborg's research on the transuranium elements[23] (although it had been proposed as early as 1892 by British chemist Henry Bassett).
However, in contrast to the oxyfluoride, the oxychloride could well be synthesized by igniting a solution of actinium trichloride in hydrochloric acid with ammonia.
The most concentrated actinium sample prepared from raw material consisted of 7 micrograms of 227Ac in less than 0.1 milligrams of La2O3, and complete separation was never achieved.
After the synthesis, actinium is separated from radium and from the products of decay and nuclear fusion, such as thorium, polonium, lead and bismuth.
[39] An alternative procedure is anion exchange with an appropriate resin in nitric acid, which can result in a separation factor of 1,000,000 for radium and actinium vs. thorium in a two-stage process.
Actinium can then be separated from radium, with a ratio of about 100, using a low cross-linking cation exchange resin and nitric acid as eluant.
[43] 225Ac was first produced artificially at the Institute for Transuranium Elements (ITU) in Germany using a cyclotron and at St George Hospital in Sydney using a linac in 2000.
[44] This rare isotope has potential applications in radiation therapy and is most efficiently produced by bombarding a radium-226 target with 20–30 MeV deuterium ions.
The oxide of 227Ac pressed with beryllium is also an efficient neutron source with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs.
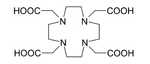
The major difficulty with application of 225Ac was that intravenous injection of simple actinium complexes resulted in their accumulation in the bones and liver for a period of tens of years.
As a result, after the cancer cells were quickly killed by alpha particles from 225Ac, the radiation from the actinium and its daughters might induce new mutations.
The latter delivery combination was tested on mice and proved to be effective against leukemia, lymphoma, breast, ovarian, neuroblastoma and prostate cancers.
[51][52][53] The medium half-life of 227Ac (21.77 years) makes it a very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters.
The associated processes cannot be studied with the required accuracy by direct measurements of current velocities (of the order 50 meters per year).
[54][55] There are theoretical predictions that AcHx hydrides (in this case with very high pressure) are a candidate for a near room-temperature superconductor as they have Tc significantly higher than H3S, possibly near 250 K.[56] 227Ac is highly radioactive and experiments with it are carried out in a specially designed laboratory equipped with a tight glove box.
[57] For trace quantities, fume hoods with good aeration suffice; for gram amounts, hot cells with shielding from the intense gamma radiation emitted by 227Ac are necessary.