Coordination number
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it.
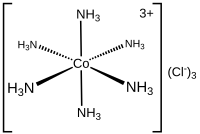
[1] For example, [Cr(NH3)2Cl2Br2]− has Cr3+ as its central cation, which has a coordination number of 6 and is described as hexacoordinate.
The coordination number does not distinguish the geometry of such complexes, i.e. octahedral vs trigonal prismatic.
For transition metal complexes, coordination numbers range from 2 (e.g., AuI in Ph3PAuCl) to 9 (e.g., ReVII in [ReH9]2−).
Metals in the f-block (the lanthanoids and actinoids) can accommodate higher coordination number due to their greater ionic radii and availability of more orbitals for bonding.
When the surrounding ligands are much smaller than the central atom, even higher coordination numbers may be possible.
[5] At the opposite extreme, steric shielding can give rise to unusually low coordination numbers.
An extremely rare instance of a metal adopting a coordination number of 1 occurs in the terphenyl-based arylthallium(I) complex 2,6-Tipp2C6H3Tl, where Tipp is the 2,4,6-triisopropylphenyl group.
Various ways exist for assigning the contribution made to the coordination number of the central iron atom by each cyclopentadienide ligand.
The contribution could be assigned as one since there is one ligand, or as five since there are five neighbouring atoms, or as three since there are three electron pairs involved.
[8] The coordination numbers are well defined for atoms in the interior of a crystal lattice: one counts the nearest neighbors in all directions.
[10] A common way to determine the coordination number of an atom is by X-ray crystallography.
[11] The coordination number of an atom can be determined straightforwardly by counting nearest neighbors.
α-Aluminium has a regular cubic close packed structure, fcc, where each aluminium atom has 12 nearest neighbors, 6 in the same plane and 3 above and below and the coordination polyhedron is a cuboctahedron.
α-Iron has a body centered cubic structure where each iron atom has 8 nearest neighbors situated at the corners of a cube.
[13] In some cases a different definition of coordination number is used that includes atoms at a greater distance than the nearest neighbours.
The very broad definition adopted by the International Union of Crystallography, IUCR, states that the coordination number of an atom in a crystalline solid depends on the chemical bonding model and the way in which the coordination number is calculated.
Regular hexagonal close packing of spheres would predict that each atom has 12 nearest neighbours and a triangular orthobicupola (also called an anticuboctahedron or twinned cuboctahedron) coordination polyhedron.
[15] Similar considerations can be applied to the regular body centred cube structure where in addition to the 8 nearest neighbors there 6 more, approximately 15% more distant,[12] and in this case the coordination number is often considered to be 14.
The nickel ions are 6-coordinate with a distorted octahedral coordination polyhedron where columns of octahedra share opposite faces.
Other compounds that share this structure, or a closely related one are some transition metal sulfides such as FeS and CoS, as well as some intermetallics.
The octahedra around the titanium atoms share edges and vertices to form a 3-D network.
Alternative definitions for the coordination number can be found in literature, but in essence the main idea is the same.